Desiccant
From Sciencemadness Wiki
A desiccant is a chemical which is hygroscopic enough to absorb water from hydrated compounds in the same sealed environment.
Common desiccants
- Calcium
- Calcium chloride
- Calcium oxide
- Concentrated sulfuric acid
- Copper sulfate (anhydrous)
- Lanthanide chlorides and nitrates
- Magnesium sulfate
- Phosphorus pentoxide
- Silica gel
- Sodium and other alkali metals
- Sodium hydroxide
- Sodium oxide
Comparison
| Substance1 | pH | Water capacity | Effectiveness | Reversible | Notes |
|---|---|---|---|---|---|
| Acetonitrile | High | High | Yes | Rarely used | |
| Activated alumina | Basic or acidic | Medium | High | Yes | Can also be used to adsorb fluorides |
| Activated charcoal | Medium | Medium | Yes | Will also adsorb other gasses | |
| Aerogel | High | High | Yes | Expensive | |
| Aluminium nitrate | Medium | Medium | No | ||
| Bentonite clay | |||||
| Benzophenone | Often used in combination with sodium or potassium | ||||
| Cadmium nitrate | |||||
| Calcium | High | Very high | No | Reaction with water releases large amounts of hydrogen | |
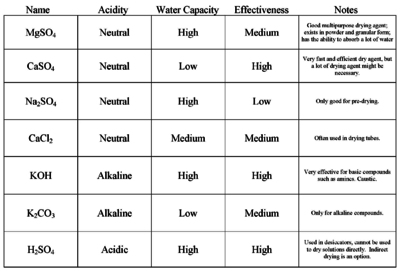
| Calcium chloride | Neutral | High | Medium | Yes | Deliquescent; often used in drying tubes |
| Calcium hydride | High | Very high | No | ||
| Calcium nitrate | Neutral | Medium | Medium | Yes | |
| Calcium oxide | Basic | High | High | No | |
| Calcium sulfate | Neutral | Low | High | Yes | Very fast and efficient drying agent, but a lot of drying agent might be necessary |
| Cement (Portland) | Alkaline | Medium | Medium | No | Used in desiccators, cannot be used directly |
| Cerium(III) chloride | No | ||||
| Cerium(III) nitrate | No | ||||
| Cesium | No | Expensive; reaction with water is highly explosive | |||
| Cobalt(II) chloride | Yes | Mostly used as water indicator | |||
| Copper(II) sulfate | Neutral | Low | Medium | Yes | Mostly used as water indicator |
| Dysprosium(III) chloride | No | ||||
| Dysprosium(III) nitrate | No | ||||
| Erbium(III) chloride | No | ||||
| Erbium(III) nitrate | No | ||||
| Europium(III) chloride | No | ||||
| Europium(III) nitrate | No | ||||
| Gadolinium(III) chloride | No | ||||
| Gadolinium(III) nitrate | No | ||||
| Holmium(III) chloride | No | ||||
| Holmium(III) nitrate | No | ||||
| Lanthanum(III) chloride | No | ||||
| Lanthanum(III) nitrate | No | ||||
| Lithium | High | High | No | Expensive; reaction with water releases hydrogen; least violent reaction of all alkaline metals | |
| Lithium bromide | Neutral | High | High | Yes | |
| Lithium chloride | Neutral | Yes | Drying must be done in a stream of hydrogen chloride | ||
| Luthetium(III) chloride | No | ||||
| Luthetium(III) nitrate | No | ||||
| Magnesium | No | Reaction is very slow, rarely used | |||
| Magnesium sulfate | Neutral | High | Medium | Yes | Good multipurpose drying agent; exists in powder and granular form; has the ability to absorb a lot of water |
| Magnesium chloride | Neutral | High | Medium | Yes | Deliquescent |
| Molecular sieves | High | High | Yes | ||
| Neodymium(III) chloride | No | ||||
| Neodymium(III) nitrate | No | ||||
| Phosphorus pentoxide | No | ||||
| Potassium | No | More often used to remove traces of water from aprotic solvents | |||
| Potassium carbonate | Alkaline | Low | Medium | Yes | Only for alkaline compounds |
| Potassium hydroxide | Alkaline | High | High | Yes | Very effective for basic compounds, such as amines; caustic |
| Praseodymium(III) chloride | No | ||||
| Praseodymium(III) nitrate | No | ||||
| Rubidium | No | Expensive; reaction with water is highly explosive | |||
| Samarium(III) chloride | No | ||||
| Samarium(III) nitrate | No | ||||
| Silica gel | Weak acidic | Yes | |||
| Sodium | No | More often used to remove traces of water from aprotic solvents | |||
| Sodium hydroxide | Alkaline | High | High | Yes | Very effective for basic compounds, such as amines; caustic |
| Sodium oxide | No | ||||
| Sodium sulfate | Neutral | High | Low | Yes | Used to dry solvents; Requires lots of it; only good for predrying; |
| Sulfur trioxide | High | Very high | No | Tends to form a mist of sulfuric acid in contact with moist air | |
| Sulfuric acid (concentrated) | Acidic | High | High | No | Used in desiccators, cannot be used to dry solutions directly |
| Terbium(III) chloride | No | ||||
| Terbium(III) nitrate | No | ||||
| Thulium(III) chloride | No | ||||
| Thulium(III) nitrate | No | ||||
| Ytterbium(III) chloride | No | ||||
| Ytterbium(III) nitrate | No | ||||
| Yttrium(III) chloride | No | ||||
| Yttrium(III) nitrate | No | ||||
| Zinc chloride | Acidic | Yes | Drying must be done in a stream of hydrogen chloride |
1All compounds are considered anhydrous.