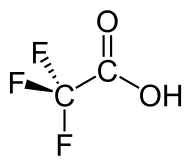
Trifluoroacetic acid
| |
| Names | |
|---|---|
| IUPAC name
Trifluoroacetic acid
| |
| Preferred IUPAC name
Trifluoroacetic acid | |
| Other names
2,2,2-Trifluoroacetic acid
2,2,2-Trifluoroethanoic acid Perfluoroacetic acid Trifluoroethanoic acid TFA | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C2HF3O2 CF3COOH | |
| Molar mass | 114.02 g/mol |
| Appearance | Colorless liquid |
| Odor | Strong pungent odor |
| Density | 1.531 g/cm3 (20 °C) 1.489 g/cm3 (25 °C) |
| Melting point | −15.4 °C (4.3 °F; 257.8 K) |
| Boiling point | 72.4 °C (162.3 °F; 345.5 K) |
| Miscible | |
| Solubility | Reacts with alkali, amines Miscible with acetone, benzene, carbon tetrachloride, diethyl ether, ethanol, hexane |
| Vapor pressure | 82.5 mmHg (20 °C) |
| Acidity (pKa) | 0.23 |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
200 mg/kg (rat, oral) |
| Related compounds | |
| Related compounds
|
Acetic acid Fluoroacetic acid Difluoroacetic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trifluoroacetic acid (TFA) is an organofluorine compound, an organic acid with the formula CF3COOH. TFA is an analogue of acetic acid, with the three hydrogen atoms replaced by three fluorine atoms. The acidity of TFA is approximately 34,000 times stronger than that of acetic acid due to the electronegativity of the trifluoromethyl group.
Contents
Properties
Chemical
Trifluoroacetic acid reacts with bases to form trifluoroacetate salts.
- CF3COOH + MOH → CF3COOM + H2O
Decarboxylation of trifluoroacetic acid yields fluoroform.[1]
Physical
Trifluoroacetic acid is a colorless volatile liquid, with a strong pungent acetic smell. It reacts with bases and amines, while miscible with water and organic solvents.
Availability
TFA is sold by various chemical suppliers.
Preparation
TFA can be synthesized by the electrofluorination of acetyl chloride and acetic anhydride, followed by hydrolysis of the resulting trifluoroacetyl fluoride:
- CH3COCl + 4 HF → CF3COF + 3 H2 + HCl
- CF3COF + H2O → CF3COOH + HF
An older route to TFA involves the oxidation of 1,1,1-trifluoro-2,3,3-trichloropropene with potassium permanganate, the latter being prepared by Swarts fluorination of hexachloropropene.
Oxidation of 1,1,1-trifluoroethane aka R-143a, a freon commonly used as refrigerent and propellant in canned air products will also yield TFA. The oxidation is done by mixing R-143a with air and passing the mixture through an electric discharge of 15,000 V. Water vapor is used as carrying medium, which also helps preventing the degradation of TFA and unreacted R-143a.[2]
Projects
- Make copper (II) trifluoroacetate
- Make trifluoroacetic anhydride
- Make 2,2,2-trifluoroethanol
- Make holmium trifluoroacetate
- Compound collecting
Handling
Safety
TFA is corrosive and should be handled with proper protection, in well ventilated areas.
Storage
Trifluoroacetic acid should be kept in closed bottles, away from heat and light, in a corrosive acid cabinet. Don't store it near ammonia or other volatile chemicals.
Disposal
Trifluoroacetic acid can be neutralized with a base. Trifluoroacetates have low toxicity and generally do not require special disposal.
References
- ↑ Kirschner, E., Chemical and Engineering News 1994, p. 8
- ↑ http://pubs.acs.org/doi/abs/10.1021/jo01062a068
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Fluorine compounds
- Organofluorine compounds
- Acids
- Strong acids
- Carboxylic acids
- Solvents
- Polar solvents
- Protic solvents
- Volatile chemicals
- Liquids
- Irritants