Ethylene
| |
| Names | |
|---|---|
| IUPAC name
Ethene
| |
| Systematic IUPAC name
Ethene | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
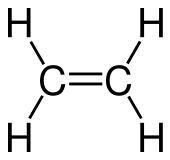
| C2H4 H2C=CH2 | |
| Molar mass | 28.05 g/mol |
| Appearance | Colorless gas |
| Odor | Sweet |
| Density | 1.178 kg/m3 (15 °C) |
| Melting point | −169.2 °C (−272.6 °F; 104.0 K) |
| Boiling point | −103.7 °C (−154.7 °F; 169.5 K) |
| 2.9 mg/L | |
| Solubility | Soluble in acetone, benzene, diethyl ether |
| Solubility in ethanol | 4.22 mg/L |
| Vapor pressure | 5.21·104 mmHg (25 °C) |
| Acidity (pKa) | 44 |
| Thermochemistry | |
| Std molar
entropy (S |
219.32 J·K−1·mol−1 |
| Std enthalpy of
formation (ΔfH |
52.47 kJ/mol |
| Hazards | |
| Safety data sheet | Praxair |
| Flash point | −136 °C (−213 °F; 137 K) |
| Related compounds | |
| Related compounds
|
Ethane Acetylene |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ethylene or ethene is a hydrocarbon which has the formula C2H4 or H2C=CH2. It is the simplest alkene.
Contents
Properties
Chemical
Ethylene is extremely flammable and will burn in an oxygen atmosphere.
- C2H4 + 3 O2 → 2 CO2 + 2 H2O
Hydration of ethene yields ethanol.
Oxidation with peracids gives ethylene oxide.
Physical
Ethylene is a colorless gas at standard conditions, lighter than air, with a sweet odor and taste.
Availability
Ethylene is sold by gas companies in gas cylinders, as compressed gas.
Preparation
Ethylene can be prepared by dehydrating ethanol in the gas phase using aluminium oxide as the catalyst.
- CH3CH2OH → : C2H4 + H2O
A more accessible way is to heat ethanol with concentrated sulfuric or phosphoric acid above 180 °C. Diethyl ether appears as a side product if the temperature is too low.
Destructive distillation of HDPE will produce lots of alkanes and alkenes, including ethylene.
Projects
- Make ethylbenzene
- Make ethylene oxide
- Make polyethylene
- Plant hormone for the ripening of fruits
Handling
Safety
Ethylene is very flammable and mixtures with air can be explosive. High concentrations in closed chambers pose an asphyxiating hazard, though being lighter than air, ethylene will not build-up in closed chambers easily.
Ethylene has anesthetic effects at high concentrations.
Storage
Ethylene tanks must be kept in a cold place, away from light, heat, and corrosive vapors.
Disposal
Ethylene can be burned or released in the air.
References
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Hydrocarbons
- Alkenes
- Gases