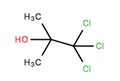
Chlorobutanol
 Chlorobutanol in a vial, with sublimated crystals
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,1,1-Trichloro-2-methylpropan-2-ol
| |||
| Properties | |||
| C4H6Cl3OH | |||
| Molar mass | 177.458 | ||
| Appearance | Off-white, waxy crystalline solid | ||
| Melting point | 95–99 °C (203–210 °F; 368–372 K) | ||
| Boiling point | 167 °C (333 °F; 440 K) | ||
| Slightly soluble | |||
| Solubility in acetone | Soluble | ||
| Related compounds | |||
| Related compounds
|
Chloral hydrate Chloroform tert-Butanol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Chlorobutanol, or 1,1,1-Trichloro-2-methyl-2-propanol, is an organic compound with a strong odor and sedative properties.
Contents
Properties
Chemical
Chlorobutanol can act as a sedative and hypnotic in mild doses, as well as an antibacterial/anti-fungal agent in low concentrations in solution. It also appears to be a powerful local anesthetic.
Physical
Chlorobutanol is a waxy or fine crystalline solid and is volatile enough to partially vaporize at low temperatures. It is known for its very strong smell, which lies somewhere between camphor and menthol. This odor is the primary reason for synthesis of chlorobutanol in the first place. It is poorly soluble in water, but soluble in acetone. It has a melting point between 95–99 °C and boils at 167°C.
Availability
Chlorobutanol is sometimes available as 0.5% - 0.05% solutions in pharmacies, but the use has declined over the years.
Preparation
Chlorobutanol can be easily prepared by reacting roughly 9 parts acetone and 1 part chloroform at low temperatures over a long period. This reaction is catalyzed by a very necessary addition of a small amount of sodium hydroxide or potassium hydroxide. The synthesis takes place over a number of hours and can be achieved at the temperature of most household freezers. After the reaction has been allowed to progress substantially the mixture should be added to freezing water to precipitate the product. In the case that any unreacted chloroform remains, much of the produced chlorobutanol will be dissolved in it, actually allowing for an easier extraction. A re-crystallization can be done using acetone or methanol, though care must be taken to prevent sublimation of the chlorobutanol. A better way to purify chlorobutanol is to first dry it with a desiccant and then intentionally sublimate it in a beaker at high heat and allowing it to deposit in crystal form on the underside of a flask of ice-water.
Projects
- Odor compound collection
Handling
Safety
Ingestion of chlorobutanol leads to sedative effects, similar to chloral hydrate or chloroform. Long exposure to vapors can cause headaches and drowsiness, and exposure to the skin may cause irritation. It is also known to damage the liver.
Storage
Chlorobutanol is difficult to store, as it tends to sublime and condense on the container walls. A sealed bottle or an ampoule can be used to store this compound.
Disposal
Chlorobutanol can be reduced with a reducing agent to butanol and burned.