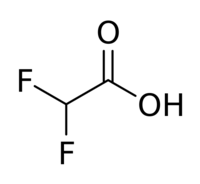
Difluoroacetic acid
From Sciencemadness Wiki
| |
| Names | |
|---|---|
| IUPAC name
2,2-Difluoroethanoic acid
| |
| Other names
2,2-Difluoroacetic acid
DFA | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C2H2F2O2 CHF2COOH | |
| Molar mass | 96.033 g/mol |
| Appearance | Colorless liquid |
| Odor | Odorless |
| Density | 1.526 g/cm3 (at 25 °C) |
| Melting point | −1 °C (30 °F; 272 K) |
| Boiling point | 132–134 °C (270–273 °F; 405–407 K) |
| Miscible | |
| Solubility | Miscible with alcohols |
| Acidity (pKa) | 1.13[1] |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 78 °C (172.4 °F; 351 K) |
| Related compounds | |
| Related compounds
|
Acetic acid Fluoroacetic acid Trifluoroacetic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Difluoroacetic acid is a chemical compound with chemical formula CHF2COOH.
Contents
Properties
Chemical
Difluoroacetic acid reacts with bases to form the corresponding difluoroacetate salts.
Physical
Difluoroacetic acid is a colorless liquid, miscible with water and alcohols.
Availability
DFA is sold by lab suppliers.
Preparation
Can be prepared by refluxing potassium fluoride with dichloroacetic acid.
Projects
- Make difluoroacetate salts
- Compound collecting
Handling
Safety
Difluoroacetic acid is toxic, though less so than its monofluoroacetic derivate. Chronic exposure has been linked to nerve and liver damage.
Storage
In closed bottles, with a PTFE seal and a hazardous chemical label.
Disposal
Should be neutralized with a base. The resulting salts should be taken to waste disposal facilities or recycled.
References
Relevant Sciencemadness threads
Categories:
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Organofluorine compounds
- Acids
- Mid-strength acids
- Carboxylic acids
- Liquids
- Irritants