Difference between revisions of "2,4-Dinitrobromobenzene"
| Line 112: | Line 112: | ||
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| − | 2,4-Dinitrobromobenzene is soluble in water and methanol. It is reactive towards reducing agents such as Sn/HCl and H<sub>2</sub>/Pd to form 2,4-diaminobromobenzene. | + | 2,4-Dinitrobromobenzene is soluble in water and [[methanol]]. It is reactive towards reducing agents such as Sn/HCl and H<sub>2</sub>/Pd to form 2,4-diaminobromobenzene. |
===Physical=== | ===Physical=== | ||
| Line 121: | Line 121: | ||
==Preparation== | ==Preparation== | ||
| − | 2,4-Dinitrobromobenzene can be prepared with relative ease in a home lab by the nitration of [[bromobenzene]]. A writeup of this reaction is available [http://www.sciencemadness.org/talk/viewthread.php?tid=28480 here]. | + | 2,4-Dinitrobromobenzene can be prepared with relative ease in a home lab by the [[nitration]] of [[bromobenzene]]. A writeup of this reaction is available [http://www.sciencemadness.org/talk/viewthread.php?tid=28480 here]. |
==Projects== | ==Projects== | ||
| Line 142: | Line 142: | ||
*[http://www.sciencemadness.org/talk/viewthread.php?tid=28480 Preparation of 2,4-dinitrobromobenzene] | *[http://www.sciencemadness.org/talk/viewthread.php?tid=28480 Preparation of 2,4-dinitrobromobenzene] | ||
| + | [[Category:Chemical compounds]] | ||
[[Category:Organic compounds]] | [[Category:Organic compounds]] | ||
[[Category:Aromatic compounds]] | [[Category:Aromatic compounds]] | ||
| − | [[Category: | + | [[Category:Nitro compounds]] |
| + | [[Category:Nitroaromatics]] | ||
[[Category:Organobromine compounds]] | [[Category:Organobromine compounds]] | ||
[[Category:Bromine compounds]] | [[Category:Bromine compounds]] | ||
[[Category:Aryl halides]] | [[Category:Aryl halides]] | ||
Revision as of 08:22, 29 August 2018
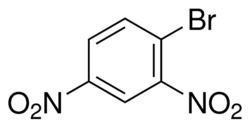 Structure of the compound
| |
| Names | |
|---|---|
| IUPAC name
1-Bromo-2,4-dinitrobenzene
| |
| Other names
O,p-Dinitrophenyl bromide
| |
| Properties | |
| C6H3BrN2O4 | |
| Molar mass | 247.004 g/mol |
| Density | 1.91 g/cm3 |
| Melting point | 70–73 °C (158–163 °F; 343–346 K) |
| Slightly soluble | |
| Solubility | Soluble in diethyl ether, ethanol, methanol |
| Hazards | |
| Safety data sheet | SantaCruzBiotechnology |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,4-Dinitrobromobenzene is a useful organic intermediate and is readily prepared in a laboratory.
Contents
Properties
Chemical
2,4-Dinitrobromobenzene is soluble in water and methanol. It is reactive towards reducing agents such as Sn/HCl and H2/Pd to form 2,4-diaminobromobenzene.
Physical
2,4-Dinitrobromobenzene, when pure, is a light yellow crystalline substance which melts at 71°C-73°C. It has the characteristic slightly sweet smell of nitroaromatic compounds, though it is toxic and inhalation of the dust should be avoided. It has a density of 1.91 g/cm3.
Availability
2,4-Dinitrobromobenzene is not offered by any suppliers available to the amateur. However, 2,4 dinitrochlorobenzene may be available from chemical suppliers and finds similar uses.
Preparation
2,4-Dinitrobromobenzene can be prepared with relative ease in a home lab by the nitration of bromobenzene. A writeup of this reaction is available here.
Projects
- Preparation of 2,4-Dinitrophenol as a precursor to DNPO
- Preparation of 2,4-Dinitrophenylhydrazine
Handling
Safety
2,4-Dinitrobromobenzene is a mutagen and skin irritant. Skin contact should be avoided by wearing gloves as some people possess a severe allergy to the compound, resulting in contact dermatitis of varying severity. It may have shock sensitivity, though the extent of which is currently undocumented.
Storage
In closed glass bottles, away from any flame source or light.
Disposal
It can be destroyed with Fenton's reagent. The best way is to dissolve it in a solvent and add it dropwise to the Fenton solution, to limit splashing and aerosolization of the compound.