Difference between revisions of "Tartrazine"
(More information) |
|||
| Line 52: | Line 52: | ||
| BoilingPtC = | | BoilingPtC = | ||
| BoilingPt_ref = | | BoilingPt_ref = | ||
| − | | BoilingPt_notes = | + | | BoilingPt_notes = Decomposes |
| − | | Density = | + | | Density = 1.9 g/cm<sup>3</sup><ref>https://www.mpbio.com/product.php?pid=05218628&country=175</ref> |
| Formula = C<sub>16</sub>H<sub>9</sub>N<sub>4</sub>Na<sub>3</sub>O<sub>9</sub>S<sub>2</sub> | | Formula = C<sub>16</sub>H<sub>9</sub>N<sub>4</sub>Na<sub>3</sub>O<sub>9</sub>S<sub>2</sub> | ||
| HenryConstant = | | HenryConstant = | ||
| Line 59: | Line 59: | ||
| MolarMass = 534.36 | | MolarMass = 534.36 | ||
| MeltingPt = | | MeltingPt = | ||
| − | | MeltingPtC = | + | | MeltingPtC = 250 |
| MeltingPt_ref = | | MeltingPt_ref = | ||
| − | | MeltingPt_notes = | + | | MeltingPt_notes = (decomposes)<ref>http://www.chemicalland21.com/lifescience/foco/TARTRAZINE.htm</ref> |
| Odor = Odorless | | Odor = Odorless | ||
| pKa = | | pKa = | ||
| Line 124: | Line 124: | ||
===Physical=== | ===Physical=== | ||
| − | Tartrazine is | + | Tartrazine is an odorless yellow solid compound, soluble in water and glycols. Some sources claim its color is either yellow-orange or (dark) orange, even red, though this is most likely from air oxidation or other impurities. |
Tartrazine's maximum absorbance in aqueous solution is 425 nm.<ref>Jain, Rajeev; Bhargava, Meenakshi; Sharma, Nidhi (2003). "Electrochemical Studies on a Pharmaceutical Azo Dye: Tartrazine". Industrial & Engineering Chemistry Research. 42 (2): 243–247. doi:10.1021/ie020228q</ref> | Tartrazine's maximum absorbance in aqueous solution is 425 nm.<ref>Jain, Rajeev; Bhargava, Meenakshi; Sharma, Nidhi (2003). "Electrochemical Studies on a Pharmaceutical Azo Dye: Tartrazine". Industrial & Engineering Chemistry Research. 42 (2): 243–247. doi:10.1021/ie020228q</ref> | ||
Latest revision as of 15:17, 15 May 2018
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
| |
| Names | |
|---|---|
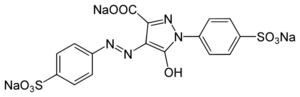
| Systematic IUPAC name
Trisodium 5-hydroxy-1-(4-sulfonatophenyl)-4-[(E)-(4-sulfonatophenyl)diazenyl]-1H-pyrazole-3-carboxylate | |
| Other names
Acid Yellow 23
E102 FD&C Yellow 5 | |
| Properties | |
| C16H9N4Na3O9S2 | |
| Molar mass | 534.36 |
| Appearance | Bright yellow solid |
| Odor | Odorless |
| Density | 1.9 g/cm3[1] |
| Melting point | 250 °C (482 °F; 523 K) (decomposes)[4] |
| Boiling point | Decomposes |
| 20 g/100 ml (25 °C) | |
| Solubility | Soluble in conc. sulfuric acid Slightly soluble in ethanol |
| Solubility in ethanol | 0.08 g/100 ml (25 °C)[2] |
| Solubility in glycerol | 18 g/100 ml (25 °C)[3] |
| Solubility in 2-methoxyethanol | 0.2 g/100 ml |
| Solubility in propylene glycol | 7 g/100 ml (25 °C) |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
12.750 mg/kg (mouse, oral) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tartrazine, commonly known as FD&C Yellow 5 in the United States, and E102 in the European Union, is a bright yellow azo dye that is one of the most common yellow food colorants.
Contents
[hide]Properties
Chemical
Tartrazine is highly soluble in water as the trisodium salt, though the free acid is very insoluble in water.
Physical
Tartrazine is an odorless yellow solid compound, soluble in water and glycols. Some sources claim its color is either yellow-orange or (dark) orange, even red, though this is most likely from air oxidation or other impurities.
Tartrazine's maximum absorbance in aqueous solution is 425 nm.[5]
Availability
While tartrazine is present in a wide variety of foods, including being the sole colorant of many brands of pickles and Lemon-Lime flavored Gatorade, the amounts that it is used in are so small that it is not worth isolating from these sources. Readily available yellow food coloring will often contain tartrazine, though it will usually be mixed with Sunset Yellow FCF (FD&C Yellow 6). These two dyes exhibit similar chemical properties, and thus would be difficult to separate, though it may be possible to do so by careful pH adjustment.
Preparation
Tartrazine can be prepared in a multiple step procedure starting with sulfanilic acid and sodium diethyl oxaloacetate.
Projects
- Yellow dye
Handling
Safety
Tartrazine is safe to handle, though it should not be consumed in macroscopic amounts. The amounts used in food are very small. It will not penetrate the skin, but it will stain the surface yellow.
Storage
Tartrazine is very stable and can be stored in virtually any airtight container at room temperature.
Disposal
Tartrazine can be disposed of by washing it down the drain with copious amounts of water.
References
- Jump up ↑ https://www.mpbio.com/product.php?pid=05218628&country=175
- Jump up ↑ Green FJ; p. 678 in The Sigma-Aldrich Handbook of Stains, Dyes, and Indicators. Milwaukee, WI: Aldrich Chem Co. (1990)
- Jump up ↑ Marmion D; Kirk-Othmer Encyclopedia of Chemical Technology. (2001). New York, NY: John Wiley & Sons; Colorants for Foods. Online Posting Date: September 14, 2007
- Jump up ↑ http://www.chemicalland21.com/lifescience/foco/TARTRAZINE.htm
- Jump up ↑ Jain, Rajeev; Bhargava, Meenakshi; Sharma, Nidhi (2003). "Electrochemical Studies on a Pharmaceutical Azo Dye: Tartrazine". Industrial & Engineering Chemistry Research. 42 (2): 243–247. doi:10.1021/ie020228q