Difference between revisions of "Potassium antimony tartrate"
(Created page with "{{Chembox | Name =Potassium antimony tartrate | Reference = | IUPACName = | PIN = | SystematicName = | OtherNames = {{Unbulleted list | tartar emetic | ''name2'' ... |...") |
(No difference)
|
Revision as of 16:19, 6 September 2015

| |
| |
| Properties | |
|---|---|
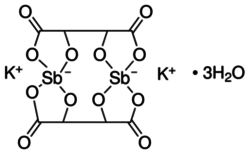
| K2Sb2(C4H2O6)2 • 3 H2O | |
| Molar mass | 667.87 g/mol |
| Appearance | White, triangular crystals |
| 83 g/L at 0ºC 333 g/L at 100ºC | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potassium antimony tartrate is a double salt containing potassium and antimony(III) cations and tartrate anions. It has the formula K2Sb2(C4H2O6)2 • 3 H2O
Contents
Properties
Chemical
Physical
Potassium antimony tartrate is a white, crystalline compound that crystallizes as flat, triangular crystals.
Availability
Potassium antimony tartrate is used sometimes by biologists studying animal diets to induce vomiting in captured animals. It is available in some developing countries as a quack treatment for alcoholism and other maladies.
Preparation
Potassium antimony tartrate is easily prepared by heating a slurry containing a stoichiometric ratio of potassium bitartrate and antimony trioxide in water to reflux for 15 to 30 minutes. At this time, most if not all of the solid should have dissolved, leaving a colorless solution. The solution is then filtered to remove any remaining reactants, and cooled in the fridge or freezer. As the solution cools, white, triangular crystals of potassium antimony tartrate form. See the relevant thread for a more detailed description.
Wikipedia claims that this compound can also be made from tartaric acid and antimony trioxide, but this is clearly false, as there would be no potassium ions present.
Projects
Handling
Safety
Potassium antimony tartrate is toxic due to its antimony content, and will induce persistent vomiting if ingested.
Storage
This compound must be stored away from children and pets due to its toxicity. It may be stored with general reagents in the lab.
Disposal
Antimony compounds are toxic to the environment, and should be disposed of as hazardous waste.
References
X-ray crystallography study of potassium antimony tartrate
CRC Handbook, 66th Edition (solubility info)
Wikipedia