Difference between revisions of "Juglone"
| Line 142: | Line 142: | ||
===Disposal=== | ===Disposal=== | ||
| − | + | Indigenous bacteria found in the soil of black walnut roots, most notably Pseudomonas putida J1, are able to metabolize juglone and use it as their primary source of energy and carbon. Because of this, juglone is not so active as a cytotoxin in well-aerated soils.<ref>[https://link.springer.com/article/10.1007/BF01012522 Schmidt, S.K. (1988). "Degradation of juglone by soil bacteria". Journal of Chemical Ecology. 14 (7): 1561–1571]</ref> | |
| + | |||
| + | Can also be neutralized with an oxidizing mixture like [[Fenton's reagent]]. | ||
==References== | ==References== | ||
Latest revision as of 18:00, 6 August 2023
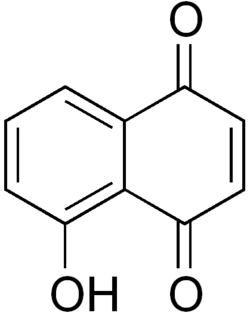 Juglone structure
| |
| Names | |
|---|---|
| IUPAC name
5-hydroxy-1,4-naphthalenedione
| |
| Other names
5-Hydroxy-1,4-naphthoquinone
5-Hydroxy-p-naphthoquinone 5-Hydroxynaphthoquinone C.I. 75500 C.I. Natural Brown 7 NCI 2323 Nucin Oil Red BS Regianin | |
| Properties | |
| C10H6O3 | |
| Molar mass | 174.155 g/mol |
| Appearance | Yellow solid |
| Density | 1.42 g/cm3 (at 25 °C)[1] |
| Melting point | 162–163 °C (324–325 °F; 435–436 K) |
| Boiling point | 381–385 °C (718–725 °F; 654–658 K) (decomposes) |
| 0.0052 g/100 ml[2] | |
| Solubility | Soluble in acetone, benzene, chloroform, diethyl ether, dioxane, DMSO, ethanol |
| Vapor pressure | 0.9 mmHg at 25 °C |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 201.3 °C (394.34 °F; 474 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
112 mg/kg (rat, oral) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Juglone (IUPAC: 5-hydroxy-1,4-naphthalenedione) is an organic compound with the molecular formula C10H6O3.
Contents
[hide]Properties
Chemical
Reduction of juglone yields hydroxyjuglone, which is the compound found in the walnut plant. Juglone appears when hydroxyjuglone oxidizes in air.
Aqueous solutions of juglone with alkali turn purpleish.[3]
Physical
Juglone is a yellowish solid, insoluble in water but more soluble in organic solvents.
Availability
Juglone occurs naturally in the leaves, roots, husks, fruit, and bark of plants (but not in the edible kernel) in the Juglandaceae family, like the common walnut, though it occurs in higher concentration in the black walnut (Juglans nigra). The average amount of juglone found in walnuts is between 2-4% by fresh weight.[4][5] Juglone is extracted from unripe walnut hulls with diethyl ether. The extract is left in air to dry and oxidize. Juglone is purified via sublimation.[6]
Pure juglone can be purchased from lab suppliers.
Preparation
Juglone can be synthesized by oxidation of the nontoxic hydrojuglone, 1,5-dihydroxynaphthalene.
Projects
- Make dye and ink
- Biodegradable herbicide
Handling
Safety
Juglone is harmful if ingested. It is highly toxic to many insect herbivores. Juglone will also stain the skin.
The compound is investigated in medicine due to its antitumoral and antimicrobial properties.
Storage
Juglone should be kept in closed bottles, away from air and other contaminants.
Disposal
Indigenous bacteria found in the soil of black walnut roots, most notably Pseudomonas putida J1, are able to metabolize juglone and use it as their primary source of energy and carbon. Because of this, juglone is not so active as a cytotoxin in well-aerated soils.[7]
Can also be neutralized with an oxidizing mixture like Fenton's reagent.
References
- Jump up ↑ Borovikov; Sivachek; Makovetskii; Novikov; Borovikov; Russian Journal of General Chemistry; vol. 67; nb. 6; (1997); p. 936 - 941
- Jump up ↑ https://link.springer.com/article/10.1007/BF00982309
- Jump up ↑ Merck, 1997
- Jump up ↑ https://archive.org/stream/bulletindelasoc181frangoog#page/n810/mode/2up
- Jump up ↑ https://www.notulaebotanicae.ro/index.php/nbha/article/view/4624
- Jump up ↑ https://www.sciencedirect.com/science/article/pii/B978938030854850006X
- Jump up ↑ Schmidt, S.K. (1988). "Degradation of juglone by soil bacteria". Journal of Chemical Ecology. 14 (7): 1561–1571