Difference between revisions of "Anthranilic acid"
| Line 11: | Line 11: | ||
| ImageAlt = | | ImageAlt = | ||
| ImageName = | | ImageName = | ||
| − | | ImageFile1 = | + | | ImageFile1 = Anthranilic acid structure.png |
| − | | ImageSize1 = | + | | ImageSize1 = 280 |
| ImageAlt1 = | | ImageAlt1 = | ||
| ImageName1 = | | ImageName1 = | ||
| + | | ImageCaption1 = Anthranilic acid structure | ||
| ImageFile2 = | | ImageFile2 = | ||
| ImageSize2 = | | ImageSize2 = | ||
Latest revision as of 17:43, 30 June 2020
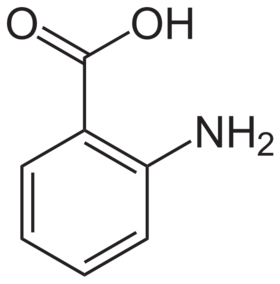 Anthranilic acid structure
| |
| Names | |
|---|---|
| IUPAC name
2-Aminobenzoic acid
| |
| Preferred IUPAC name
2-Aminobenzoic acid | |
| Systematic IUPAC name
2-Aminobenzenecarboxylic acid | |
| Other names
o-Aminobenzoic acid
Anthranilic acid Vitamin L1 | |
| Properties | |
| C7H7NO2 C6H4(NH2)(COOH) | |
| Molar mass | 137.138 g/mol |
| Appearance | White solid |
| Density | 1.412 g/cm3 (20 °C) |
| Melting point | 146–148 °C (295–298 °F; 419–421 K) |
| Boiling point | 200 °C (392 °F; 473 K) (sublimes) |
| 0.450 g/100 ml (20 °C) 0.572 g/100 ml (25 °C) | |
| Solubility | Very soluble in chloroform, dichloromethane, pyridine Soluble in diethyl ether, ethanol, methanol Slightly soluble in benzene, trifluoroacetic acid |
| Vapor pressure | 0.1 Pa (52.6 °C) |
| Acidity (pKa) | 2.17 (carboxyl; H2O) 4.85 (amino; H2O) |
| Thermochemistry | |
| Std enthalpy of
formation (ΔfH |
-380.4 KJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
4,549 mg/kg mg/kg (rat, oral) |
| Related compounds | |
| Related compounds
|
Salicylic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Anthranilic acid (o-aminobenzoic acid, 2-aminobenzoic acid, 2-AA, 2AA, AA) is an aromatic acid with the formula C6H4(NH2)(COOH). As a result of containing both acidic and basic functional groups, the compound is amphoteric.
Contents
Properties
Chemical
Decarboxylation of anthranillic acid yields aniline.[1]
Physical
Anthranilic acid is a white solid, soluble in organic solvents. It has a sweetish taste.
Availability
Anthranilic acid is sold by chemical suppliers.
Anthranilic acid is DEA List I Chemical because of its use in making the now-widely outlawed euphoric sedative drug methaqualone (Quaalude, Mandrax).
Preparation
Anthranilic acid can be produced by aminating phthalic anhydride in the presence of NaOH. The resulting sodium salt of phthalamic acid is decarbonylated via a Hofmann rearrangement of the amide group, induced by hypochlorite to anthranilic acid.
Can also be produced from phthalimide and sodium hypochloride/hypobromite.[2][3]
Projects
- Make azo dyes
- Make saccharin
- Make methyl anthranilate
- Make anthranilate-based insect repellents
Handling
Safety
Anthranilic acid chas low toxicity.
Storage
In closed bottles.
Disposal
Can be diluted and poured down the drain.
References
- ↑ https://www.nrcresearchpress.com/doi/pdf/10.1139/v52-065
- ↑ https://www.prepchem.com/synthesis-of-anthranilic-acid/
- ↑ https://www.erowid.org/archive/rhodium/chemistry/anthranilic.html