Difference between revisions of "Kojic acid"
| Line 54: | Line 54: | ||
| BoilingPt_ref = | | BoilingPt_ref = | ||
| BoilingPt_notes = (decomposes) | | BoilingPt_notes = (decomposes) | ||
| − | | Density = | + | | Density = 1.58 g/cm<sup>3</sup> (20 °C)<ref>[https://pubs.acs.org/doi/10.1021/jf502159m Li, Ying; Teng, Zi; Parkin, Kirk L.; Wang, Qin; Zhang, Qingli; Luo, Wei; Ma, Deyun; Zhao, Mouming; Journal of Agricultural and Food Chemistry; vol. 62; nb. 33; (2014); p. 8392 - 8401]</ref> |
| Formula = C<sub>6</sub>H<sub>6</sub>O<sub>4</sub> | | Formula = C<sub>6</sub>H<sub>6</sub>O<sub>4</sub> | ||
| HenryConstant = | | HenryConstant = | ||
Revision as of 20:36, 23 July 2019
 OTC kojic acid, slightly impure.
| |
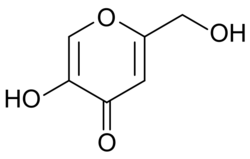 Chemical structure of kojic acid.
| |
| Names | |
|---|---|
| IUPAC name
5-Hydroxy-2-(hydroxymethyl)-4H-pyran-4-one
| |
| Other names
2-Hydroxymethyl-5-hydroxy-γ-pyrone
5-Hydroxy-2-(hydroxymethyl)-4-pyrone | |
| Properties | |
| C6H6O4 | |
| Molar mass | 142.11 g/mol |
| Appearance | White solid |
| Odor | Tree bark-like |
| Density | 1.58 g/cm3 (20 °C)[1] |
| Melting point | 153.5 °C (308.3 °F; 426.6 K) [3] |
| Boiling point | 180 °C (356 °F; 453 K) (decomposes) |
| 9.23 g/100 ml (20 °C)[2] | |
| Solubility | Soluble in acetone, DMSO, ethanol, methanol Slightly soluble in benzene, chloroform, diethyl ether, ethyl acetate, pyridine |
| Vapor pressure | 3.21·10-6 mmHg at 25 °C |
| Acidity (pKa) | 9.40 |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
1,000 mg/kg (rat, oral) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Kojic acid is an organic compound, a chelation agent produced by several species of fungi, especially Aspergillus oryzae, known in Japan as koji ("steamed rice"), from which the said compound got its name. Chemically, kojic acid is a γ-pyrone.
Contents
Properties
Chemical
Kojic acid forms a bright red complex with ferric ions. The red color persists even at a dilution 1:200,000. This property permits the estimation of kojic acid by colorimetry.[4]
Salts of kojic acid are called kojates. Kojic acid forms stable complexes of metal kojates via reaction of kojic acid with metal acetate salts such as tin, beryllium, zinc, copper, nickel, cobalt, iron, manganese, chromium, gold, palladium, indium, gallium, vanadium, and aluminium. Tris(kojic acid)aluminium(III) and -gallium(III) complexes have lipid solubility and have been shown to cross the blood-brain barrier in tests.
Physical
Kojic acid is a colorless solid. It is soluble in water and a few organic solvents, like acetone, ethanol and methanol.[5] Impure samples appear slight beige or yellowish.
Availability
Kojic acid is sold by soapmaking suppliers and other natural care shops. Can be cheaply bought online.
Preparation
Kojic acid is a by-product in the fermentation process of malting rice caused by the Aspergillus oryzae fungus, process used in the manufacturing of sake, the Japanese rice wine.
Purification an be done by recrystallization from acetone, ethanol-ether, and methanol-ethyl acetate. Sublimation at low pressure, between 150–200 °C is another route.
Projects
- Make metal complexes
- Make red complex with Fe3+
- Gravimetric determination of copper
- Food additive to prevent browning of food
- Make skin lightning products
Handling
Safety
Kojic acid doesn't appear to be toxic. In acute, chronic, reproductive and genotoxicity studies, kojic acid was not found to pose high toxicity. Due to slow absorption into the circulation from human skin, it does not reach the threshold at tumor promotion and weak carcinogenicity effects were only suspected.
Kojic acid it's a slight irritant due to its ability to lighten skin.
Storage
In closed bottles, away from oxidizers.
Disposal
No special disposal is required, can be dumped in trash or down the drain.
References
- ↑ Li, Ying; Teng, Zi; Parkin, Kirk L.; Wang, Qin; Zhang, Qingli; Luo, Wei; Ma, Deyun; Zhao, Mouming; Journal of Agricultural and Food Chemistry; vol. 62; nb. 33; (2014); p. 8392 - 8401
- ↑ http://www.t3db.ca/toxins/T3D3769
- ↑ Lide, D.R. CRC Handbook of Chemistry and Physics 88TH Edition 2007-2008. CRC Press, Taylor & Francis, Boca Raton, FL 2007, p. 3-292
- ↑ T. Yabuta, Orig. Com. 8th Intern. Congr. Appl. Chem. (Appendix), 26, 455 (1912); Chem. Abstracts, 7, 2191 (1913)
- ↑ https://www.sciencedirect.com/science/article/pii/S0096533208601186