Difference between revisions of "Titration"
m |
|||
| Line 7: | Line 7: | ||
=== Process === | === Process === | ||
| − | Titration begins with first making a standard solution of a known substance, next, the solution is filled into a [[burette]], a small amount of indicator is then added to the unknown sample, if an indicator is not | + | Titration begins with first making a standard solution of a known substance, next, the solution is filled into a [[burette]], a small amount of indicator is then added to the unknown sample, if an indicator is not available, a pH or redox meter can also be used. The the point of the indicator is to determine the endpoint of the titration, where the reaction has gone to completion. The stopcock is then opened to dispense the titrant, as the solution reaches it's end point, one should reduce the rate of the titrant flow substantially as to increase the accuracy of the titration. The titration ends when the indicator changes to a different color. |
=== Types of titration === | === Types of titration === | ||
Revision as of 23:08, 16 December 2018
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
Titration or titrimetry is a common laboratory method of quantitative chemical analysis, used to determine the concentration of a known substance from a solution or chemical mixture.
Contents
Titration
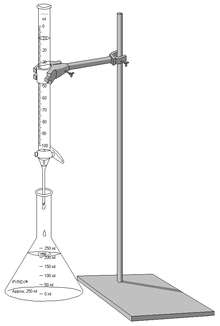
Titration is a method of quantitative chemical analysis used to determine the concentration of an unknown sample. A known concentration of a reagent is prepared (the titrant) that reacts with the substance being titrated (the titrand). The volume of titrant required to neutralize the titrand then can be used to determine the concentration of the unknown sample.
Process
Titration begins with first making a standard solution of a known substance, next, the solution is filled into a burette, a small amount of indicator is then added to the unknown sample, if an indicator is not available, a pH or redox meter can also be used. The the point of the indicator is to determine the endpoint of the titration, where the reaction has gone to completion. The stopcock is then opened to dispense the titrant, as the solution reaches it's end point, one should reduce the rate of the titrant flow substantially as to increase the accuracy of the titration. The titration ends when the indicator changes to a different color.