Difference between revisions of "1,2-Dichloroethane"
(First page migrated!) |
|||
| Line 1: | Line 1: | ||
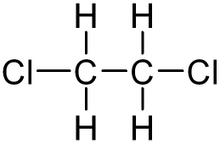
| − | [[File:1,2-Dichloroethane | + | [[File:1,2-Dichloroethane.png|thumb|220x220px|Structure of 1,2-dichloroethane]] |
'''1,2-dichloroethane''', or '''ethylene dichloride''', is a common solvent and reagent, going by the abbreviations '''EDC''' and '''1,2-DCA'''. It is also used to make [[vinyl chloride]], the major precursor to [[polyvinyl chloride]], commonly known as PVC. | '''1,2-dichloroethane''', or '''ethylene dichloride''', is a common solvent and reagent, going by the abbreviations '''EDC''' and '''1,2-DCA'''. It is also used to make [[vinyl chloride]], the major precursor to [[polyvinyl chloride]], commonly known as PVC. | ||
Revision as of 20:59, 14 June 2015
1,2-dichloroethane, or ethylene dichloride, is a common solvent and reagent, going by the abbreviations EDC and 1,2-DCA. It is also used to make vinyl chloride, the major precursor to polyvinyl chloride, commonly known as PVC.
Contents
Properties
Chemical
Ethylene dichloride is a slightly reactive polar solvent. It is sometimes used as a precursor to ethylenediamine and 1,1,1-trichloroethane[1].
Physical
Ethylene dichloride is colorless and has a high index of refraction, giving it a shiny appearance, similar to chloroform, which it also smells similarly to. It is a versatile solvent, though it does form azeotropes with water and many other solvents.
Availability
1,2-Dichloroethane is mostly bought from chemical suppliers rather than ordinary retail stores due to its inherent dangers.
Preparation
1,2-Dichloroethane can be prepared by bubbling chlorine through ethene solution (Eg. dissolved in Carbon Tetrachloride) with a Iron(III) chloride catalyst, or bubbling oxygen through a solution of 1 molar part ethene to four molar parts Hydrochloric acid with a Copper(II) chloride catalyst. The first procedure produces pure 1,2-dichloroethane, while the second results in water as well[2].
Projects
- Ethylenediamine synthesis
- Make PVC
Safety
Ethylene dichloride is toxic (by inhalation), flammable, and a carcinogen. All these hazards are amplified by the chemical's volatile nature. This chemical is also unstable when in the presence of aluminium, zinc, and iron[3].
References
- ↑ http://en.wikipedia.org/wiki/1,2-Dichloroethane
- ↑ http://en.wikipedia.org/wiki/1,2-Dichloroethane
- ↑ http://en.wikipedia.org/wiki/1,2-Dichloroethane