Difference between revisions of "Niacin"
(Fixed grammar and spelling, added SMILES code and added image.) |
|||
| Line 115: | Line 115: | ||
Decarboxylation of niacin gives [[pyridine]] | Decarboxylation of niacin gives [[pyridine]] | ||
| − | :C<sub>6</sub>H<sub>5</sub>NO<sub>2</sub> → C<sub>5</sub>H<sub>5</sub>N + CO<sub>2</sub> | + | : C<sub>6</sub>H<sub>5</sub>NO<sub>2</sub> → C<sub>5</sub>H<sub>5</sub>N + CO<sub>2</sub> |
[[Copper chromite]] is used as a catalyst. | [[Copper chromite]] is used as a catalyst. | ||
Latest revision as of 21:54, 15 March 2021
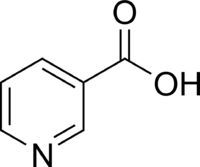
| |
| Names | |
|---|---|
| IUPAC name
Pyridine-3-carboxylic acid
| |
| Other names
Bionic
Nicotinic acid Vitamin B3 | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C6H5NO2 | |
| Molar mass | 123.1094 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.473 g/cm3 |
| Melting point | 237 °C (459 °F; 510 K) |
| Boiling point | Sublimes |
| 1.8 g/100 ml (20 °C) | |
| Solubility | Soluble in DMSO Slightly soluble in diethyl ether, ethanol Insoluble in lipids |
| Vapor pressure | 5.7·10-6 mmHg °C |
| Acidity (pKa) | 4.75 |
| Thermochemistry | |
| Std enthalpy of
formation (ΔfH |
−344.9 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 193 °C |
| Related compounds | |
| Related compounds
|
Dipicolinic acid Pyridine |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Niacin, also known as nicotinic acid or vitamin B3, is an organic compound with the formula C6H5NO2. It is an important essential human nutrient.
Contents
Properties
Chemical
Decarboxylation of niacin gives pyridine
- C6H5NO2 → C5H5N + CO2
Copper chromite is used as a catalyst.
Physical
Niacin is a white solid, slightly soluble in water, but more so in organic solvents.
Availability
Niacin can be easily found in various Vitamin B3 supplements. To extract it, simply crush the niacin in a mortar or better in a coffee grinder. Add sodium hydroxide to convert it into the more soluble sodium nicotinate, and vacuum filter the solution. Convert it back to nicotinic acid by carefully adding hydrochloric acid. Since niacin is poorly soluble in water, it will precipitate out of the solution. The resulting precipitate is filtered, however, this is difficult to do, as niacin water suspensions are non-Newtonian fluids, similar to starch and water mixtures. The resulting precipitate should be air-dried, as vacuum drying doesn't work very well. The yield from this method is about 50-70%. Using an organic solvent will improve the yield.[1]
Niacin however is best purchased in bulk, rather than extracted from pills. It can be purchased online relatively cheap.
Preparation
Niacin is best purchased than prepared.
It can be isolated from the oxidation of nicotine. Nitric acid is commonly used.
Projects
- Make pyridine
- Make arecoline
Handling
Safety
Niacin is edible, but avoid consuming lab-grade nicotinic acid.
Storage
Niacin should be stored in closed bottles.
Disposal
No special disposal is required. Discard it as you wish.
References
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Carboxylic acids
- Pyridine derivatives
- Biologically-derived compounds
- Acids
- Aromatic compounds
- Readily available chemicals