Difference between revisions of "Difluoroacetic acid"
From Sciencemadness Wiki
(Added image, SMILES code and slightly more information about reaction with bases.) |
|||
| Line 7: | Line 7: | ||
| OtherNames = 2,2-Difluoroacetic acid<br>DFA | | OtherNames = 2,2-Difluoroacetic acid<br>DFA | ||
<!-- Images --> | <!-- Images --> | ||
| − | | ImageFile = | + | | ImageFile = Difluoroacetic acid structural formula.png |
| ImageSize = | | ImageSize = | ||
| ImageAlt = | | ImageAlt = | ||
| Line 43: | Line 43: | ||
| 3DMet = | | 3DMet = | ||
| Abbreviations = | | Abbreviations = | ||
| − | | SMILES = | + | | SMILES = C(C(=O)O)(F)F |
}} | }} | ||
| Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| Line 112: | Line 112: | ||
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| − | Difluoroacetic acid reacts with bases. | + | Difluoroacetic acid reacts with bases to form the corresponding [[difluoroacetate]] salts. |
===Physical=== | ===Physical=== | ||
Revision as of 22:52, 24 September 2020
| |
| Names | |
|---|---|
| IUPAC name
2,2-Difluoroethanoic acid
| |
| Other names
2,2-Difluoroacetic acid
DFA | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C2H2F2O2 CHF2COOH | |
| Molar mass | 96.033 g/mol |
| Appearance | Colorless liquid |
| Odor | Odorless |
| Density | 1.526 g/cm3 (at 25 °C) |
| Melting point | −1 °C (30 °F; 272 K) |
| Boiling point | 132–134 °C (270–273 °F; 405–407 K) |
| Miscible | |
| Solubility | Miscible with alcohols |
| Acidity (pKa) | 1.13[1] |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 78 °C (172.4 °F; 351 K) |
| Related compounds | |
| Related compounds
|
Acetic acid Fluoroacetic acid Trifluoroacetic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
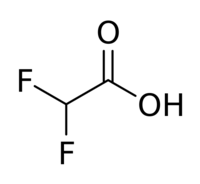
Difluoroacetic acid is a chemical compound with chemical formula CHF2COOH.
Contents
Properties
Chemical
Difluoroacetic acid reacts with bases to form the corresponding difluoroacetate salts.
Physical
Difluoroacetic acid is a colorless liquid, miscible with water and alcohols.
Availability
DFA is sold by lab suppliers.
Preparation
Can be prepared by refluxing KF with dichloroacetic acid.
Projects
- Make difluoroacetate salts
Handling
Safety
Difluoroacetic acid is toxic, though less so than its monofluoroacetic derivate. Chronic exposure has been linked to nerve and liver damage.
Storage
In closed bottles, with a PTFE seal and a hazardous chemical label.
Disposal
Should be neutralized with a base. The resulting salts should be taken to waste disposal facilities or recycled.
References
Relevant Sciencemadness threads
Categories:
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Organofluorine compounds
- Acids
- Mid-strength acids
- Carboxylic acids
- Liquids
- Irritants