Difference between revisions of "Lead picrate"
m |
(Expansion) |
||
| Line 6: | Line 6: | ||
| PIN = | | PIN = | ||
| SystematicName = 2,4-Dinitro-6-(oxo{[(2,4,6-trinitrophenoxy)-λ2-plumbanyl]oxy}ammonio)phenolate | | SystematicName = 2,4-Dinitro-6-(oxo{[(2,4,6-trinitrophenoxy)-λ2-plumbanyl]oxy}ammonio)phenolate | ||
| − | | OtherNames = | + | | OtherNames = Lead dipicrate<br>Lead picrate<br>Lead salt of trinitrophenol |
| − | + | ||
| − | + | ||
| − | + | ||
<!-- Images --> | <!-- Images --> | ||
| ImageFile = leadpicrate.jpeg | | ImageFile = leadpicrate.jpeg | ||
| − | | ImageSize = | + | | ImageSize = 300px |
| ImageAlt = | | ImageAlt = | ||
| + | | ImageCaption = Freshly prepared and dried basic lead picrate. | ||
| ImageName = Basic Lead Picrate | | ImageName = Basic Lead Picrate | ||
| ImageFile1 = PbP.png | | ImageFile1 = PbP.png | ||
| Line 57: | Line 55: | ||
| BoilingPt_notes = | | BoilingPt_notes = | ||
| Density = | | Density = | ||
| − | | Formula = | + | | Formula = C<sub>12</sub>H<sub>4</sub>N<sub>6</sub>O<sub>14</sub>Pb |
| HenryConstant = | | HenryConstant = | ||
| LogP = | | LogP = | ||
| Line 65: | Line 63: | ||
| MeltingPt_ref = | | MeltingPt_ref = | ||
| MeltingPt_notes = | | MeltingPt_notes = | ||
| + | | Odor = Odorless | ||
| pKa = | | pKa = | ||
| pKb = | | pKb = | ||
| − | | Solubility = | + | | Solubility = Barely soluble in water at 20°C |
| SolubleOther = | | SolubleOther = | ||
| Solvent = | | Solvent = | ||
| − | | VaporPressure = | + | | VaporPressure = ~0 mmHg |
}} | }} | ||
| Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| Line 85: | Line 84: | ||
}} | }} | ||
| Section5 = {{Chembox Explosive | | Section5 = {{Chembox Explosive | ||
| − | | ShockSens = | + | | ShockSens = Moderately shock sensitive |
| − | | FrictionSens = | + | | FrictionSens = Highly friction sensitive |
| DetonationV = | | DetonationV = | ||
| REFactor = | | REFactor = | ||
}} | }} | ||
| Section6 = {{Chembox Hazards | | Section6 = {{Chembox Hazards | ||
| − | | AutoignitionPt = | + | | AutoignitionPt = Detonates |
| ExploLimits = | | ExploLimits = | ||
| − | | ExternalMSDS = | + | | ExternalMSDS = None |
| − | | FlashPt = | + | | FlashPt = Detonates |
| LD50 = | | LD50 = | ||
| LC50 = | | LC50 = | ||
| Line 108: | Line 107: | ||
| OtherFunction = | | OtherFunction = | ||
| OtherFunction_label = | | OtherFunction_label = | ||
| − | | OtherCompounds = [[Picric acid]] | + | | OtherCompounds = [[Picric acid]]<br>[[Lead styphnate]]<br>[[Lead(II) azide]] |
}} | }} | ||
}} | }} | ||
| − | |||
'''Lead picrate''', or more properly, '''basic lead picrate''', is an energetic, toxic, and explosive lead salt. | '''Lead picrate''', or more properly, '''basic lead picrate''', is an energetic, toxic, and explosive lead salt. | ||
== Properties == | == Properties == | ||
=== Physical === | === Physical === | ||
| − | |||
Lead picrate is an orange, very dense non-hygroscopic lead compound. Its melting and boiling point are both unknown, as it tends to deflagrate or detonate before it reaches such a temperature. | Lead picrate is an orange, very dense non-hygroscopic lead compound. Its melting and boiling point are both unknown, as it tends to deflagrate or detonate before it reaches such a temperature. | ||
=== Chemical === | === Chemical === | ||
| + | |||
| + | === Explosive === | ||
| + | Lead picrate is moderate sensitive to shock and heat, but has high friction sensitivity. | ||
| + | |||
| + | == Availability == | ||
| + | Lead picrate is not sold by any supplier, due to its instability. | ||
| + | |||
| + | Old reagent bottles containing picric acid that have lead in their lid will accumulate small amounts of lead picrate, which may be set off if one tries to open the bottle. | ||
| + | |||
| + | == Preparation == | ||
| + | Lead picrate can be made by adding lead oxide to picric acid, in a solution.<ref>http://www.powerlabs.org/chemlabs/lead_picrate.htm</ref> | ||
| + | |||
| + | Manufacturing, storing and handling this compound may require an explosive permit, depending on the country. | ||
| + | |||
| + | == Projects == | ||
| + | *Amateur detonators | ||
| + | |||
| + | == Handling == | ||
| + | === Safety === | ||
| + | Lead picrate is extremely toxic. It is also a sensitive explosive, which is higher if the material is dry. | ||
| + | |||
| + | === Storage === | ||
| + | Due to its sensitivity, lead picrate should not be stored in large amounts. Wet powder is less sensitive than dry material. | ||
| + | |||
| + | === Disposal === | ||
| + | Small amounts of this material can be neutralized via controlled detonation. However, the disposal of larger amounts is complicated. One way is to add the material in water, where it's slowly and gently oxidized using various oxidizing solutions, like [[Fenton's reagent]], ozone, or some other oxidizer. The final product will contain insoluble lead oxide precipitate, which is toxic, and needs to be either recovered or taken to a hazardous waste disposal center. | ||
| + | |||
| + | == References == | ||
| + | <references/> | ||
| + | === Relevant Sciencemadness threads === | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=328 Lead Picrate] | ||
| + | |||
| + | [[Category:Chemical compounds]] | ||
| + | [[Category:Organic compounds]] | ||
| + | [[Category:Aromatic compounds]] | ||
| + | [[Category:Phenols]] | ||
| + | [[Category:Nitroaromatics]] | ||
| + | [[Category:Lead compounds]] | ||
| + | [[Category:Energetic materials]] | ||
| + | [[Category:Primary explosives]] | ||
| + | [[Category:Things that should NOT be messed with except by professionals]] | ||
| + | [[Category:Things that can kill you very quickly]] | ||
Revision as of 18:43, 15 February 2019
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
 Freshly prepared and dried basic lead picrate.
| |
| |
| Names | |
|---|---|
| IUPAC name
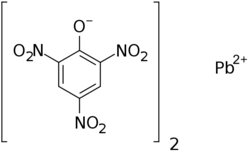
lead(2+);2,4,6-trinitrophenolate
| |
| Systematic IUPAC name
2,4-Dinitro-6-(oxo{[(2,4,6-trinitrophenoxy)-λ2-plumbanyl]oxy}ammonio)phenolate | |
| Other names
Lead dipicrate
Lead picrate Lead salt of trinitrophenol | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C12H4N6O14Pb | |
| Molar mass | 663.4g/mol |
| Appearance | Dense, orange powder |
| Odor | Odorless |
| Melting point | Detonates |
| Boiling point | Detonates |
| Barely soluble in water at 20°C | |
| Vapor pressure | ~0 mmHg |
| Hazards | |
| Safety data sheet | None |
| Flash point | Detonates |
| Related compounds | |
| Related compounds
|
Picric acid Lead styphnate Lead(II) azide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lead picrate, or more properly, basic lead picrate, is an energetic, toxic, and explosive lead salt.
Contents
Properties
Physical
Lead picrate is an orange, very dense non-hygroscopic lead compound. Its melting and boiling point are both unknown, as it tends to deflagrate or detonate before it reaches such a temperature.
Chemical
Explosive
Lead picrate is moderate sensitive to shock and heat, but has high friction sensitivity.
Availability
Lead picrate is not sold by any supplier, due to its instability.
Old reagent bottles containing picric acid that have lead in their lid will accumulate small amounts of lead picrate, which may be set off if one tries to open the bottle.
Preparation
Lead picrate can be made by adding lead oxide to picric acid, in a solution.[1]
Manufacturing, storing and handling this compound may require an explosive permit, depending on the country.
Projects
- Amateur detonators
Handling
Safety
Lead picrate is extremely toxic. It is also a sensitive explosive, which is higher if the material is dry.
Storage
Due to its sensitivity, lead picrate should not be stored in large amounts. Wet powder is less sensitive than dry material.
Disposal
Small amounts of this material can be neutralized via controlled detonation. However, the disposal of larger amounts is complicated. One way is to add the material in water, where it's slowly and gently oxidized using various oxidizing solutions, like Fenton's reagent, ozone, or some other oxidizer. The final product will contain insoluble lead oxide precipitate, which is toxic, and needs to be either recovered or taken to a hazardous waste disposal center.
References
Relevant Sciencemadness threads
- Article stubs
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Aromatic compounds
- Phenols
- Nitroaromatics
- Lead compounds
- Energetic materials
- Primary explosives
- Things that should NOT be messed with except by professionals
- Things that can kill you very quickly