Difference between revisions of "Chlorobutanol"
m (Added picture captions) |
Diachrynic (Talk | contribs) |
||
| (6 intermediate revisions by 2 users not shown) | |||
| Line 5: | Line 5: | ||
| PIN = | | PIN = | ||
| SystematicName = | | SystematicName = | ||
| − | | OtherNames = | + | | OtherNames = |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
<!-- Images --> | <!-- Images --> | ||
| − | | ImageFile = | + | | ImageFile = Chlorobutanol_sublimed.jpg |
| ImageSize = 250 | | ImageSize = 250 | ||
| ImageAlt = | | ImageAlt = | ||
| − | | ImageName = | + | | ImageName = |
| − | | ImageCaption = | + | | ImageCaption = Crystals of chlorobutanol obtained by sublimation |
| ImageFile1 = | | ImageFile1 = | ||
| ImageSize1 = | | ImageSize1 = | ||
| Line 58: | Line 53: | ||
| BoilingPt_ref = | | BoilingPt_ref = | ||
| BoilingPt_notes = | | BoilingPt_notes = | ||
| − | | Density = | + | | Density = 1.404 g/cm<sup>3</sup> |
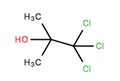
| Formula = C<sub>4</sub>H<sub>6</sub>Cl<sub>3</sub>OH | | Formula = C<sub>4</sub>H<sub>6</sub>Cl<sub>3</sub>OH | ||
| HenryConstant = | | HenryConstant = | ||
| LogP = | | LogP = | ||
| − | | MolarMass = | + | | MolarMass = 177.458 |
| MeltingPt = | | MeltingPt = | ||
| MeltingPtC = 95-99 | | MeltingPtC = 95-99 | ||
| Line 71: | Line 66: | ||
| Solubility = Slightly soluble | | Solubility = Slightly soluble | ||
| SolubleOther = Soluble | | SolubleOther = Soluble | ||
| − | | Solvent = [[ | + | | Solvent = [[acetone]] |
| VaporPressure = | | VaporPressure = | ||
}} | }} | ||
| Line 95: | Line 90: | ||
| AutoignitionPt = | | AutoignitionPt = | ||
| ExploLimits = | | ExploLimits = | ||
| − | | ExternalMSDS = | + | | ExternalMSDS = [https://www.docdroid.net/H5yEdWL/chlorobutanol-sa.pdf.html Sigma-Aldrich] |
| FlashPt = | | FlashPt = | ||
| LD50 = | | LD50 = | ||
| LC50 = | | LC50 = | ||
| − | | MainHazards = | + | | MainHazards = Irritant |
| NFPA-F = | | NFPA-F = | ||
| NFPA-H = | | NFPA-H = | ||
| Line 110: | Line 105: | ||
| OtherFunction = | | OtherFunction = | ||
| OtherFunction_label = | | OtherFunction_label = | ||
| − | | OtherCompounds = [[Chloral hydrate]] | + | | OtherCompounds = [[Chloral hydrate]]<br>[[Chloroform]]<br>[[tert-Butanol]] |
}} | }} | ||
}} | }} | ||
| Line 120: | Line 115: | ||
===Physical=== | ===Physical=== | ||
| − | Chlorobutanol is a waxy or fine crystalline solid and is volatile enough to partially vaporize at low temperatures. It is known for its very strong smell, which lies somewhere between camphor and menthol. This odor is the primary reason for synthesis of chlorobutanol in the first place. It is poorly soluble in water, but soluble in acetone. It has a melting point between 95–99 °C and boils at 167°C. | + | Chlorobutanol is a waxy or fine crystalline solid and is volatile enough to partially vaporize at low temperatures. It is known for its very strong smell, which lies somewhere between camphor and [[menthol]]. This odor is the primary reason for synthesis of chlorobutanol in the first place. It is poorly soluble in water, but soluble in acetone. It has a melting point between 95–99 °C and boils at 167°C. |
==Availability== | ==Availability== | ||
| − | Chlorobutanol is sometimes available as 0.5% - 0.05% solutions in pharmacies, but the use has declined over the years. | + | Chlorobutanol is sometimes available as 0.5% - 0.05% solutions in pharmacies, but the use has declined over the years, making it harder to find in some places. |
==Preparation== | ==Preparation== | ||
Chlorobutanol can be easily prepared by reacting roughly 9 parts [[acetone]] and 1 part [[chloroform]] at low temperatures over a long period. This reaction is catalyzed by a very necessary addition of a small amount of [[sodium hydroxide]] or [[potassium hydroxide]]. The synthesis takes place over a number of hours and can be achieved at the temperature of most household freezers. After the reaction has been allowed to progress substantially the mixture should be added to freezing water to precipitate the product. In the case that any unreacted chloroform remains, much of the produced chlorobutanol will be dissolved in it, actually allowing for an easier extraction. A re-crystallization can be done using acetone or methanol, though care must be taken to prevent sublimation of the chlorobutanol. A better way to purify chlorobutanol is to first dry it with a desiccant and then intentionally sublimate it in a beaker at high heat and allowing it to deposit in crystal form on the underside of a flask of ice-water. | Chlorobutanol can be easily prepared by reacting roughly 9 parts [[acetone]] and 1 part [[chloroform]] at low temperatures over a long period. This reaction is catalyzed by a very necessary addition of a small amount of [[sodium hydroxide]] or [[potassium hydroxide]]. The synthesis takes place over a number of hours and can be achieved at the temperature of most household freezers. After the reaction has been allowed to progress substantially the mixture should be added to freezing water to precipitate the product. In the case that any unreacted chloroform remains, much of the produced chlorobutanol will be dissolved in it, actually allowing for an easier extraction. A re-crystallization can be done using acetone or methanol, though care must be taken to prevent sublimation of the chlorobutanol. A better way to purify chlorobutanol is to first dry it with a desiccant and then intentionally sublimate it in a beaker at high heat and allowing it to deposit in crystal form on the underside of a flask of ice-water. | ||
| + | |||
| + | ==Projects== | ||
| + | *Odor compound collection | ||
==Handling== | ==Handling== | ||
| Line 137: | Line 135: | ||
===Disposal=== | ===Disposal=== | ||
Chlorobutanol can be reduced with a reducing agent to butanol and burned. | Chlorobutanol can be reduced with a reducing agent to butanol and burned. | ||
| + | |||
| + | ==Gallery== | ||
| + | <gallery widths="200" position="center" columns="4" orientation="none"> | ||
| + | Chlorobutanol.jpg|Chlorobutanol in a vial, with sublimated crystals | ||
| + | </gallery> | ||
==References== | ==References== | ||
| Line 149: | Line 152: | ||
[[Category:Alcohols]] | [[Category:Alcohols]] | ||
[[Category:Tertiary alcohols]] | [[Category:Tertiary alcohols]] | ||
| + | [[Category:Halohydrins]] | ||
[[Category:Fragrant compounds]] | [[Category:Fragrant compounds]] | ||
[[Category:Volatile chemicals]] | [[Category:Volatile chemicals]] | ||
Latest revision as of 07:29, 14 February 2021
 Crystals of chlorobutanol obtained by sublimation
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,1,1-Trichloro-2-methylpropan-2-ol
| |||
| Properties | |||
| C4H6Cl3OH | |||
| Molar mass | 177.458 | ||
| Appearance | Off-white, waxy crystalline solid | ||
| Density | 1.404 g/cm3 | ||
| Melting point | 95–99 °C (203–210 °F; 368–372 K) | ||
| Boiling point | 167 °C (333 °F; 440 K) | ||
| Slightly soluble | |||
| Solubility in acetone | Soluble | ||
| Hazards | |||
| Safety data sheet | Sigma-Aldrich | ||
| Related compounds | |||
| Related compounds
|
Chloral hydrate Chloroform tert-Butanol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Chlorobutanol, or 1,1,1-Trichloro-2-methyl-2-propanol, is an organic compound with a strong odor and sedative properties.
Contents
Properties
Chemical
Chlorobutanol can act as a sedative and hypnotic in mild doses, as well as an antibacterial/anti-fungal agent in low concentrations in solution. It also appears to be a powerful local anesthetic.
Physical
Chlorobutanol is a waxy or fine crystalline solid and is volatile enough to partially vaporize at low temperatures. It is known for its very strong smell, which lies somewhere between camphor and menthol. This odor is the primary reason for synthesis of chlorobutanol in the first place. It is poorly soluble in water, but soluble in acetone. It has a melting point between 95–99 °C and boils at 167°C.
Availability
Chlorobutanol is sometimes available as 0.5% - 0.05% solutions in pharmacies, but the use has declined over the years, making it harder to find in some places.
Preparation
Chlorobutanol can be easily prepared by reacting roughly 9 parts acetone and 1 part chloroform at low temperatures over a long period. This reaction is catalyzed by a very necessary addition of a small amount of sodium hydroxide or potassium hydroxide. The synthesis takes place over a number of hours and can be achieved at the temperature of most household freezers. After the reaction has been allowed to progress substantially the mixture should be added to freezing water to precipitate the product. In the case that any unreacted chloroform remains, much of the produced chlorobutanol will be dissolved in it, actually allowing for an easier extraction. A re-crystallization can be done using acetone or methanol, though care must be taken to prevent sublimation of the chlorobutanol. A better way to purify chlorobutanol is to first dry it with a desiccant and then intentionally sublimate it in a beaker at high heat and allowing it to deposit in crystal form on the underside of a flask of ice-water.
Projects
- Odor compound collection
Handling
Safety
Ingestion of chlorobutanol leads to sedative effects, similar to chloral hydrate or chloroform. Long exposure to vapors can cause headaches and drowsiness, and exposure to the skin may cause irritation. It is also known to damage the liver.
Storage
Chlorobutanol is difficult to store, as it tends to sublime and condense on the container walls. A sealed bottle or an ampoule can be used to store this compound.
Disposal
Chlorobutanol can be reduced with a reducing agent to butanol and burned.