Difference between revisions of "Triethylamine"
| (9 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | {{Chembox | |
| − | '''Triethylamine''' is an [[amine]], with the formula '''N( | + | | Name = Triethylamine |
| + | | Reference = | ||
| + | | IUPACName = | ||
| + | | PIN = N,N-Diethylethanamine | ||
| + | | SystematicName = | ||
| + | | OtherNames = (Diethylamino)ethane<br>(Triethyl)amine | ||
| + | <!-- Images --> | ||
| + | | ImageFile = | ||
| + | | ImageSize = | ||
| + | | ImageAlt = | ||
| + | | ImageName = | ||
| + | | ImageFile1 = Triethylamine distilled by NurdRage.jpg | ||
| + | | ImageSize1 = 300 | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageCaption1 = Freshly distilled triethylamine | ||
| + | | ImageFile2 = Triethylamine_structure.gif | ||
| + | | ImageSize2 = 250 | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageCaption2 = Structure of triethylamine | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = | ||
| + | | ImageSizeL1 = | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| + | | Section1 = {{Chembox Identifiers | ||
| + | | 3DMet = | ||
| + | | Abbreviations = | ||
| + | | SMILES = | ||
| + | }} | ||
| + | | Section2 = {{Chembox Properties | ||
| + | | AtmosphericOHRateConstant = | ||
| + | | Appearance = Colorless liquid | ||
| + | | BoilingPt = | ||
| + | | BoilingPtC = 88.8 | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = | ||
| + | | Density = 0.7255 g/cm<sup>3</sup> | ||
| + | | Formula = C<sub>6</sub>H<sub>15</sub>N<br>N(CH<sub>2</sub>CH<sub>3</sub>)<sub>3</sub><br>NEt<sub>3</sub> | ||
| + | | HenryConstant = 66 μmol·Pa<sup>−1</sup>·kg<sup>−1</sup> | ||
| + | | LogP = 1.647 | ||
| + | | MolarMass = 101.19 g/mol | ||
| + | | MeltingPt = | ||
| + | | MeltingPtC = −114.7 | ||
| + | | MeltingPt_ref = | ||
| + | | MeltingPt_notes = | ||
| + | | Odor = Fishy | ||
| + | | pKa = | ||
| + | | pKb = | ||
| + | | Solubility = Miscible (<18.7 °C)<br>5.5 g/100 ml (20 °C)<br>6.86 g/100 ml (25 °C) | ||
| + | | SolubleOther = Miscible with [[acetone]], [[diethyl ether]], [[ethanol]]<br>Soluble in [[benzene]], [[carbon tetrachloride]], [[chloroform]], mineral and natural oils | ||
| + | | Solvent = | ||
| + | | VaporPressure = 6.899–8.506 kPa | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = | ||
| + | | CrystalStruct = | ||
| + | | MolShape = | ||
| + | }} | ||
| + | | Section4 = {{Chembox Thermochemistry | ||
| + | | DeltaGf = | ||
| + | | DeltaHc = −4,377.63 to −4,376.55 kJ/mol | ||
| + | | DeltaHf = −169 kJ/mol | ||
| + | | Entropy = | ||
| + | | HeatCapacity = 216.43 J·K<sup>−1</sup>·mol<sup>−1</sup> | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = | ||
| + | | FrictionSens = | ||
| + | | DetonationV = | ||
| + | | REFactor = | ||
| + | }} | ||
| + | | Section6 = {{Chembox Hazards | ||
| + | | AutoignitionPt = 312 °C (594 °F; 585 K) | ||
| + | | ExploLimits = 1.2–8% | ||
| + | | ExternalMSDS = [https://www.docdroid.net/PwjoUX4/triethylamine-sa.pdf.html Sigma-Aldrich] | ||
| + | | FlashPt = −15 °C (5 °F; 258 K) | ||
| + | | LD50 = 580 mg/kg (rabbit, dermal)<br>730 mg/kg (rat, oral) | ||
| + | | LC50 = | ||
| + | | MainHazards = Harmful | ||
| + | | NFPA-F = | ||
| + | | NFPA-H = | ||
| + | | NFPA-R = | ||
| + | | NFPA-S = | ||
| + | }} | ||
| + | | Section7 = {{Chembox Related | ||
| + | | OtherAnions = | ||
| + | | OtherCations = | ||
| + | | OtherFunction = | ||
| + | | OtherFunction_label = | ||
| + | | OtherCompounds = [[Ethylamine]]<br>[[Diethylamine]] | ||
| + | }} | ||
| + | }} | ||
| + | '''Triethylamine''' is an [[amine]], with the formula '''N(CH<sub>2</sub>CH<sub>3</sub>)<sub>3</sub>''' or '''NEt<sub>3</sub>'''. Pure triethylamine exists as a [[liquid]] at room temperature. | ||
| + | |||
==Properties== | ==Properties== | ||
===Physical=== | ===Physical=== | ||
| − | Triethylamine is a colorless liquid with a melting point of -114. | + | Triethylamine is a colorless liquid with a melting point of -114.7 °C and a boiling point around 88.6 °C. It has a strong, fishy odor reminiscent of ammonia. |
===Chemical=== | ===Chemical=== | ||
| − | Triethylamine is a base commonly used in organic chemistry to prepare esters and amides from acyl chlorides.Like other tertiary amines,it catalyzes the formation of urethane foams and epoxy resins. | + | Triethylamine is a base commonly used in organic chemistry to prepare esters and amides from acyl chlorides. Like other tertiary amines,it catalyzes the formation of urethane foams and epoxy resins. |
| + | |||
== Availability == | == Availability == | ||
| + | It is sold by chemical suppliers. | ||
==Preparation== | ==Preparation== | ||
| − | Triethylamine can be prepared by heating ethyl bromide and anhydrous ammonia in absolute ethanol in an oven for three hours,removing the product,distilling off the alcohol,and adding hydrochloric acid to the product to convert it to its hydrochloride salt. Melt the triethylamine hydrochloride and filter off the ammonium chloride crystals that remain. After filtering,freebase the triethylamine and once again add ethyl bromide. Heat in a steam oven for three hours and allow it to slowly cool. Remove the resulting liquid. | + | There are many ways to make triethylamine, though only a few are efficient. |
| + | |||
| + | THIS SIMPLIFIED OUTLINE STILL NEEDS IMPROVEMENT. FOR A FULL PROCEDURE,SEE THE LINKED REFERENCE. | ||
| + | |||
| + | Triethylamine can be prepared by heating [[ethyl bromide]] and anhydrous [[ammonia]] in absolute [[ethanol]] in an oven for three hours, removing the product, distilling off the alcohol, and adding [[hydrochloric acid]] to the product to convert it to its hydrochloride salt. Melt the triethylamine hydrochloride and filter off the [[ammonium chloride]] crystals that remain. After filtering, freebase the triethylamine and once again add ethyl bromide. Heat in a steam oven for three hours and allow it to slowly cool. Remove the resulting liquid.<ref>http://www.sciencemadness.org/talk/files.php?pid=233855&aid=17257</ref> | ||
== Projects == | == Projects == | ||
| + | *Quaternary ammonium compounds | ||
| + | *Anesthetize mosquitoes | ||
== Handling == | == Handling == | ||
=== Safety and toxicity === | === Safety and toxicity === | ||
| + | Triethylamine is a toxic liquid. | ||
==== Legal issues ==== | ==== Legal issues ==== | ||
===Storage=== | ===Storage=== | ||
| + | Triethylamine should be stored in a special cabinet, away from any heat source. | ||
===Disposal=== | ===Disposal=== | ||
| + | Triethylamine can be diluted with a flammable solvent and burned in an open place. | ||
| + | Adding an acid will convert it to salt, which is easier to dispose of. | ||
==References== | ==References== | ||
<references/> | <references/> | ||
===Relevant Sciencemadness threads=== | ===Relevant Sciencemadness threads=== | ||
| − | http://www.sciencemadness.org/talk/ | + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=61855 Triethylamine synthesis] |
| − | + | [[Category:Chemical compounds]] | |
| + | [[Category:Organic compounds]] | ||
| + | [[Category:Nitrogen compounds]] | ||
| + | [[Category:Amines]] | ||
| + | [[Category:Alkylamines]] | ||
| + | [[Category:Tertiary amines]] | ||
| + | [[Category:Bases]] | ||
| + | [[Category:Organic bases]] | ||
| + | [[Category:Lewis bases]] | ||
| + | [[Category:Solvents]] | ||
| + | [[Category:Amide solvents]] | ||
| + | [[Category:Carcinogenic]] | ||
| + | [[Category:Foul smelling compounds]] | ||
| + | [[Category:Liquids]] | ||
Latest revision as of 14:59, 25 December 2023
 Freshly distilled triethylamine
| |
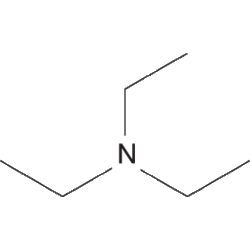 Structure of triethylamine
| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N-Diethylethanamine | |
| Other names
(Diethylamino)ethane
(Triethyl)amine | |
| Properties | |
| C6H15N N(CH2CH3)3 NEt3 | |
| Molar mass | 101.19 g/mol |
| Appearance | Colorless liquid |
| Odor | Fishy |
| Density | 0.7255 g/cm3 |
| Melting point | −114.7 °C (−174.5 °F; 158.5 K) |
| Boiling point | 88.8 °C (191.8 °F; 361.9 K) |
| Miscible (<18.7 °C) 5.5 g/100 ml (20 °C) 6.86 g/100 ml (25 °C) | |
| Solubility | Miscible with acetone, diethyl ether, ethanol Soluble in benzene, carbon tetrachloride, chloroform, mineral and natural oils |
| Vapor pressure | 6.899–8.506 kPa |
| Thermochemistry | |
| Std enthalpy of
formation (ΔfH |
−169 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | −15 °C (5 °F; 258 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
580 mg/kg (rabbit, dermal) 730 mg/kg (rat, oral) |
| Related compounds | |
| Related compounds
|
Ethylamine Diethylamine |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triethylamine is an amine, with the formula N(CH2CH3)3 or NEt3. Pure triethylamine exists as a liquid at room temperature.
Contents
Properties
Physical
Triethylamine is a colorless liquid with a melting point of -114.7 °C and a boiling point around 88.6 °C. It has a strong, fishy odor reminiscent of ammonia.
Chemical
Triethylamine is a base commonly used in organic chemistry to prepare esters and amides from acyl chlorides. Like other tertiary amines,it catalyzes the formation of urethane foams and epoxy resins.
Availability
It is sold by chemical suppliers.
Preparation
There are many ways to make triethylamine, though only a few are efficient.
THIS SIMPLIFIED OUTLINE STILL NEEDS IMPROVEMENT. FOR A FULL PROCEDURE,SEE THE LINKED REFERENCE.
Triethylamine can be prepared by heating ethyl bromide and anhydrous ammonia in absolute ethanol in an oven for three hours, removing the product, distilling off the alcohol, and adding hydrochloric acid to the product to convert it to its hydrochloride salt. Melt the triethylamine hydrochloride and filter off the ammonium chloride crystals that remain. After filtering, freebase the triethylamine and once again add ethyl bromide. Heat in a steam oven for three hours and allow it to slowly cool. Remove the resulting liquid.[1]
Projects
- Quaternary ammonium compounds
- Anesthetize mosquitoes
Handling
Safety and toxicity
Triethylamine is a toxic liquid.
Legal issues
Storage
Triethylamine should be stored in a special cabinet, away from any heat source.
Disposal
Triethylamine can be diluted with a flammable solvent and burned in an open place.
Adding an acid will convert it to salt, which is easier to dispose of.