Difference between revisions of "Actinium"
(→Physical) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 22: | Line 22: | ||
<!-- Periodic table --> | <!-- Periodic table --> | ||
|above=[[Lanthanum|La]] | |above=[[Lanthanum|La]] | ||
| − | |below= | + | |below=Ubu |
|left=[[Radium]] | |left=[[Radium]] | ||
|right=[[Thorium]] | |right=[[Thorium]] | ||
| Line 271: | Line 271: | ||
|engvar= | |engvar= | ||
}} | }} | ||
| − | Actinium is a highly radioactive, silvery-metallic element with a bluish glow due to its extreme radiation. | + | '''Actinium''' ('''Ac''') is a highly radioactive, silvery-metallic element with a bluish glow due to its extreme radiation. |
==Properties== | ==Properties== | ||
| Line 278: | Line 278: | ||
===Physical=== | ===Physical=== | ||
| − | Actinium is a soft metal, comparable to sodium in consistency. Pure actinium is silvery-white, though it quickly oxidises in air to form a white crust. It has a density of approximately 10g/cm<sup>3</sup> | + | Actinium is a soft metal, comparable to sodium in consistency. Pure actinium is silvery-white, though it quickly oxidises in air to form a white crust. It has a density of approximately 10g/cm<sup>3</sup>. Due to it's intense radiation, actinium glows a dull blue-green. |
==Availability== | ==Availability== | ||
Latest revision as of 22:06, 17 September 2022
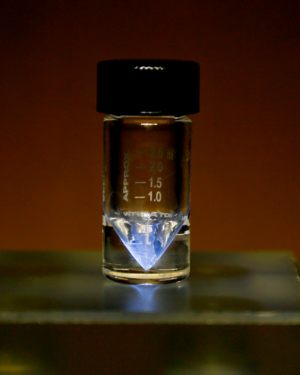 Picture of a sample of actinium in a v-vial, glowing blue due to its extreme radiation ionizing the air surrounding the sample. Oak Ridge National Laboratory, Actinium sample (31481701837), Image resized, CC BY 2.0 | |||||
| General properties | |||||
|---|---|---|---|---|---|
| Name, symbol | Actinium, Ac | ||||
| Appearance | Silvery metallic, glows lightly blue in the dark | ||||
| Actinium in the periodic table | |||||
| |||||
| Atomic number | 89 | ||||
| Standard atomic weight (Ar) | |||||
| Element category | Actinides | ||||
| Group, block | 3 (sometimes considered group n/a); d-block (sometimes considered to be f-block) | ||||
| Period | period 7 | ||||
| Electron configuration | [Rn] 6d1 7s2 | ||||
per shell | 2, 8, 18, 32, 18, 9, 2 | ||||
| Physical properties | |||||
| Phase | solid | ||||
| Melting point | 1500 K (1227 °C, 2240 °F) (estimated) | ||||
| Boiling point | 3500±300 K (3200±300 °C, 5800±500 °F) (extrapolated) | ||||
| Density near r.t. | 10 g/cm3 | ||||
| Heat of fusion | 14 kJ/mol | ||||
| Heat of | 400 kJ/mol | ||||
| Molar heat capacity | 27.2 J/(mol·K) | ||||
| Atomic properties | |||||
| Oxidation states | +3 | ||||
| Electronegativity | Pauling scale: 1.1 | ||||
| energies |
1st: 499 kJ/mol 2nd: 1170 kJ/mol 3rd: 1900 kJ/mol (more) | ||||
| Covalent radius | 215 pm | ||||
| Miscellanea | |||||
| Thermal conductivity | 12 W/(m·K) | ||||
| CAS Registry Number | 7440-34-8 | ||||
| History | |||||
| Discovery and first isolation | Friedrich Oskar Giesel (1902) | ||||
| Named by | André-Louis Debierne (1899) | ||||
Actinium (Ac) is a highly radioactive, silvery-metallic element with a bluish glow due to its extreme radiation.
Contents
Properties
Chemical
To do
Physical
Actinium is a soft metal, comparable to sodium in consistency. Pure actinium is silvery-white, though it quickly oxidises in air to form a white crust. It has a density of approximately 10g/cm3. Due to it's intense radiation, actinium glows a dull blue-green.
Availability
Due to its extreme radioactivity and the difficulty to produce it, it is only available to a select few industries for very high prices.
Isolation
Even though extremely impractical, one might be able to extract very tiny amounts of it from uranium ores. It occurs in thorium, but at so small amounts that it's basically impossible to extract enough of it to be able to collect it.
Projects
- Element collection
Handling
Safety
Actinium is extremely radioactive and emits a lot of carcinogenic and toxic radiation. Handle it in a glove box with thick gloves and walls, walls preferably of lead glass.
Storage
Store in a thick lead-walled container.
Disposal
The best method of disposal is probably to leave it in an area with a lot of natural radioactive ores.