Difference between revisions of "Lead picrate"
(Expansion) |
(→Preparation) |
||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 54: | Line 54: | ||
| BoilingPt_ref = | | BoilingPt_ref = | ||
| BoilingPt_notes = | | BoilingPt_notes = | ||
| − | | Density = | + | | Density = 2.831 g/cm<sup>3</sup> |
| + | | Density_ref = https://archive.org/stream/Megalomanias_Controversial_Chemlab_12-08-2004/Megalomanias_Controversial_Chemlab_12-08-2004_djvu.txt | ||
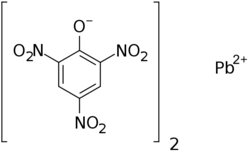
| Formula = C<sub>12</sub>H<sub>4</sub>N<sub>6</sub>O<sub>14</sub>Pb | | Formula = C<sub>12</sub>H<sub>4</sub>N<sub>6</sub>O<sub>14</sub>Pb | ||
| HenryConstant = | | HenryConstant = | ||
| Line 86: | Line 87: | ||
| ShockSens = Moderately shock sensitive | | ShockSens = Moderately shock sensitive | ||
| FrictionSens = Highly friction sensitive | | FrictionSens = Highly friction sensitive | ||
| − | | DetonationV = | + | | DetonationV = 4400 m/s |
| REFactor = | | REFactor = | ||
}} | }} | ||
| Line 117: | Line 118: | ||
=== Chemical === | === Chemical === | ||
| + | Lead picrate is a highly toxic compound; PPE should be worn whenever handling this compound. | ||
=== Explosive === | === Explosive === | ||
| Line 127: | Line 129: | ||
== Preparation == | == Preparation == | ||
| − | Lead picrate can be made by adding lead oxide to picric acid, in a solution.<ref>http://www.powerlabs.org/chemlabs/lead_picrate.htm</ref> | + | Lead picrate can be made by adding lead oxide to [[picric acid]], in a solution.<ref>http://www.powerlabs.org/chemlabs/lead_picrate.htm</ref> |
| + | |||
| + | Another method involves adding a solution of sodium hydroxide to a solution of picric acid, with a slight excess of NaOH, forming a basified [[sodium picrate]] solution. This solution is then slowly added to a near boiling solution of [[lead(II) nitrate]] with constant stirring. The mixture is then allowed to cool to room temperature, and the resultant basic lead picrate settles very quickly to the bottom due to its density. It is then filtered and washed with distilled water. It is not recommended to make much of this salt at any given time, nor is it recommended to store it dry.<ref> Youtuber AllChemystery uploaded a video on this process, but it has since been removed. However, backups of it exist and can requested on the SM forum.</ref> | ||
Manufacturing, storing and handling this compound may require an explosive permit, depending on the country. | Manufacturing, storing and handling this compound may require an explosive permit, depending on the country. | ||
| Line 139: | Line 143: | ||
=== Storage === | === Storage === | ||
| − | Due to its sensitivity, lead picrate should not be stored in large amounts. Wet powder is less sensitive than dry material. | + | Due to its sensitivity, lead picrate should not be stored in large amounts. Wet powder is less sensitive than dry material. |
=== Disposal === | === Disposal === | ||
Latest revision as of 19:35, 16 May 2023
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
 Freshly prepared and dried basic lead picrate.
| |
| |
| Names | |
|---|---|
| IUPAC name
lead(2+);2,4,6-trinitrophenolate
| |
| Systematic IUPAC name
2,4-Dinitro-6-(oxo{[(2,4,6-trinitrophenoxy)-λ2-plumbanyl]oxy}ammonio)phenolate | |
| Other names
Lead dipicrate
Lead picrate Lead salt of trinitrophenol | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C12H4N6O14Pb | |
| Molar mass | 663.4g/mol |
| Appearance | Dense, orange powder |
| Odor | Odorless |
| Density | 2.831 g/cm3 |
| Melting point | Detonates |
| Boiling point | Detonates |
| Barely soluble in water at 20°C | |
| Vapor pressure | ~0 mmHg |
| Hazards | |
| Safety data sheet | None |
| Flash point | Detonates |
| Related compounds | |
| Related compounds
|
Picric acid Lead styphnate Lead(II) azide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lead picrate, or more properly, basic lead picrate, is an energetic, toxic, and explosive lead salt.
Contents
Properties
Physical
Lead picrate is an orange, very dense non-hygroscopic lead compound. Its melting and boiling point are both unknown, as it tends to deflagrate or detonate before it reaches such a temperature.
Chemical
Lead picrate is a highly toxic compound; PPE should be worn whenever handling this compound.
Explosive
Lead picrate is moderate sensitive to shock and heat, but has high friction sensitivity.
Availability
Lead picrate is not sold by any supplier, due to its instability.
Old reagent bottles containing picric acid that have lead in their lid will accumulate small amounts of lead picrate, which may be set off if one tries to open the bottle.
Preparation
Lead picrate can be made by adding lead oxide to picric acid, in a solution.[1]
Another method involves adding a solution of sodium hydroxide to a solution of picric acid, with a slight excess of NaOH, forming a basified sodium picrate solution. This solution is then slowly added to a near boiling solution of lead(II) nitrate with constant stirring. The mixture is then allowed to cool to room temperature, and the resultant basic lead picrate settles very quickly to the bottom due to its density. It is then filtered and washed with distilled water. It is not recommended to make much of this salt at any given time, nor is it recommended to store it dry.[2]
Manufacturing, storing and handling this compound may require an explosive permit, depending on the country.
Projects
- Amateur detonators
Handling
Safety
Lead picrate is extremely toxic. It is also a sensitive explosive, which is higher if the material is dry.
Storage
Due to its sensitivity, lead picrate should not be stored in large amounts. Wet powder is less sensitive than dry material.
Disposal
Small amounts of this material can be neutralized via controlled detonation. However, the disposal of larger amounts is complicated. One way is to add the material in water, where it's slowly and gently oxidized using various oxidizing solutions, like Fenton's reagent, ozone, or some other oxidizer. The final product will contain insoluble lead oxide precipitate, which is toxic, and needs to be either recovered or taken to a hazardous waste disposal center.
References
- ↑ http://www.powerlabs.org/chemlabs/lead_picrate.htm
- ↑ Youtuber AllChemystery uploaded a video on this process, but it has since been removed. However, backups of it exist and can requested on the SM forum.
Relevant Sciencemadness threads
- Article stubs
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Aromatic compounds
- Phenols
- Nitroaromatics
- Lead compounds
- Energetic materials
- Primary explosives
- Things that should NOT be messed with except by professionals
- Things that can kill you very quickly