Difference between revisions of "Acetylsalicylic acid"
(Created page with "thumb Acetylsalicylic acid is an organic acid commonly known as aspirin. Acetylsalicylic acid (ASA) is prone to hydrolysis, breaking down into salicylic ac...") |
(Restructuring) |
||
| Line 1: | Line 1: | ||
| − | [[File:ASA.png|thumb]] | + | [[File:Acetylsalicylic acid.JPG|thumb|240px|Pure aspirin as a fluffy, crystalline powder]] |
| − | Acetylsalicylic acid is an organic acid commonly known as aspirin. | + | [[File:ASA.png|thumb|200px]] |
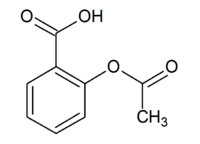
| + | '''Acetylsalicylic acid''' or '''2-(acetoxy)benzoic acid''', is an organic acid commonly known as '''aspirin'''. | ||
| + | ==Properties== | ||
| + | ===Chemical=== | ||
Acetylsalicylic acid (ASA) is prone to hydrolysis, breaking down into [[salicylic acid]] and [[acetic acid]]. Solutions of ASA are therefore not very stable and hot or boiling water will quickly break apart the molecule into the two smaller organic acids. The solid must be protected from air as moisture in the air will also degrade it. | Acetylsalicylic acid (ASA) is prone to hydrolysis, breaking down into [[salicylic acid]] and [[acetic acid]]. Solutions of ASA are therefore not very stable and hot or boiling water will quickly break apart the molecule into the two smaller organic acids. The solid must be protected from air as moisture in the air will also degrade it. | ||
| − | |||
| − | |||
| − | + | Hydrolysis of ASA can be observed through the smell of acetic acid, solutions that have undergone significant hydrolysis have a very noticeable vinegar smell to them. | |
| − | + | ||
| − | + | ||
| − | |||
| − | |||
| − | |||
Acetylsalicylic acid readily hydrolyzes in hot water or solutions that are highly acidic or basic. While this can be an obstacle for some syntheses, it can be taken advantage of to make the more stable [[salicylic acid]], which fills many of the same roles, by refluxing acetylsalicylic acid powder in low pH solution, cooling, and filtering out the crystals of salicylic acid, which should be recrystallized. | Acetylsalicylic acid readily hydrolyzes in hot water or solutions that are highly acidic or basic. While this can be an obstacle for some syntheses, it can be taken advantage of to make the more stable [[salicylic acid]], which fills many of the same roles, by refluxing acetylsalicylic acid powder in low pH solution, cooling, and filtering out the crystals of salicylic acid, which should be recrystallized. | ||
| Line 20: | Line 16: | ||
'[[Copper(II) acetylsalicylate|Copper aspirinate]]' can be formed by using the ASA as a ligand to the copper(II) ions, or simply precipitated by the mixture of solutions of [[copper(II) sulfate]] and [[sodium acetylsalicylate]]. This is discussed in detail at http://www.sciencemadness.org/talk/viewthread.php?tid=9920 | '[[Copper(II) acetylsalicylate|Copper aspirinate]]' can be formed by using the ASA as a ligand to the copper(II) ions, or simply precipitated by the mixture of solutions of [[copper(II) sulfate]] and [[sodium acetylsalicylate]]. This is discussed in detail at http://www.sciencemadness.org/talk/viewthread.php?tid=9920 | ||
| + | |||
| + | ===Physical=== | ||
| + | Aspirin is a white solid compound, soluble in [[diethyl ether]], [[acetone]] and [[ethanol]], poorly soluble in water (3 mg/mL at 20 °C), benzene and even less soluble in [[propylene glycol]]. | ||
| + | |||
| + | == Availability == | ||
| + | As one of the world's most commonly used medicine, aspirin is found in every pharmacy on earth as well as most supermarkets. | ||
| + | Even basic aspirin tablets contain some form of binder to keep it in tablet form or make it more air stable. To remove these, the tablets can be crushed and washed with denatured alcohol. The ASA is soluble in the alcohol while the binders are not, so any solids that remain undissolved can be filtered off. | ||
| + | |||
| + | The alcohol can then be carefully boiled off or evaporated away. Acetylsalicylic acid forms light, fluffy, glittering white crystals when recrystallized from solution. These crystals should be stored in a dry, airtight container to prevent hydrolysis. Some hydrolysis to salicylic acid and acetic acid is unavoidable, however, so acetylsalicylic acid cannot be kept for very long periods of time without loss in purity. | ||
| + | |||
| + | ==Preparation== | ||
| + | Acetylsalicylic acid is prepared via the esterification of salicylic acid with [[acetic anhydride]]. This reaction is performed in the presence of a small amount of catalyst, such as [[sulfuric acid]] or [[phosphoric acid]]. The reaction yields aspirin and [[acetic acid]]. The acetic acid is neutralized and the aspirin is extracted. | ||
| + | |||
| + | == Projects == | ||
| + | *Make sodium acetylsalicylate | ||
| + | *Picric acid synthesis | ||
| + | *Copper(II) acetylsalicylate synthesis | ||
| + | |||
| + | ==Handling== | ||
| + | ===Safety=== | ||
| + | Aspirin should not be consumed in large quantities, as it has anticoagulant properties and can cause gastrointestinal bleeding. Lab grade aspirin should never be consumed. | ||
| + | |||
| + | ===Storage=== | ||
| + | In a dry place to prevent hydrolysis. | ||
| + | |||
| + | ===Disposal=== | ||
| + | Aspirin can be safely poured down the drain on dumped in the trash. | ||
| + | |||
| + | ==References== | ||
| + | <references/> | ||
| + | ===Relevant Sciencemadness threads=== | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=26850 aspirin synthesis without acetyl anhydride] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=9920 Copper(II) Aspirinate Synthesis] | ||
{{DEFAULTSORT:Acetylsalicylic acid}} | {{DEFAULTSORT:Acetylsalicylic acid}} | ||
| + | |||
| + | [[Category:Chemical compounds]] | ||
[[Category:Organic compounds]] | [[Category:Organic compounds]] | ||
| + | [[Category:Aromatic compounds]] | ||
[[Category:Acids]] | [[Category:Acids]] | ||
[[Category:Salicylates]] | [[Category:Salicylates]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
[[Category:Carboxylic acids]] | [[Category:Carboxylic acids]] | ||
[[Category:Materials unstable in basic solution]] | [[Category:Materials unstable in basic solution]] | ||
[[Category:Materials unstable in acidic solution]] | [[Category:Materials unstable in acidic solution]] | ||
| + | [[Category:Ligands]] | ||
| + | [[Category:Chelating agents]] | ||
| + | [[Category:Readily available chemicals]] | ||
Revision as of 19:20, 3 September 2015
Acetylsalicylic acid or 2-(acetoxy)benzoic acid, is an organic acid commonly known as aspirin.
Contents
Properties
Chemical
Acetylsalicylic acid (ASA) is prone to hydrolysis, breaking down into salicylic acid and acetic acid. Solutions of ASA are therefore not very stable and hot or boiling water will quickly break apart the molecule into the two smaller organic acids. The solid must be protected from air as moisture in the air will also degrade it.
Hydrolysis of ASA can be observed through the smell of acetic acid, solutions that have undergone significant hydrolysis have a very noticeable vinegar smell to them.
Acetylsalicylic acid readily hydrolyzes in hot water or solutions that are highly acidic or basic. While this can be an obstacle for some syntheses, it can be taken advantage of to make the more stable salicylic acid, which fills many of the same roles, by refluxing acetylsalicylic acid powder in low pH solution, cooling, and filtering out the crystals of salicylic acid, which should be recrystallized.
ASA reacts with sodium carbonate and bicarbonate to form sodium acetylsalicylate. Sodium hydroxide cannot be used to make the sodium salt as it will hydrolyse the ASA.
Purified aspirin is the precursor in one of the most commonly used syntheses of trinitrophenol (picric acid), a dye and explosive.
'Copper aspirinate' can be formed by using the ASA as a ligand to the copper(II) ions, or simply precipitated by the mixture of solutions of copper(II) sulfate and sodium acetylsalicylate. This is discussed in detail at http://www.sciencemadness.org/talk/viewthread.php?tid=9920
Physical
Aspirin is a white solid compound, soluble in diethyl ether, acetone and ethanol, poorly soluble in water (3 mg/mL at 20 °C), benzene and even less soluble in propylene glycol.
Availability
As one of the world's most commonly used medicine, aspirin is found in every pharmacy on earth as well as most supermarkets. Even basic aspirin tablets contain some form of binder to keep it in tablet form or make it more air stable. To remove these, the tablets can be crushed and washed with denatured alcohol. The ASA is soluble in the alcohol while the binders are not, so any solids that remain undissolved can be filtered off.
The alcohol can then be carefully boiled off or evaporated away. Acetylsalicylic acid forms light, fluffy, glittering white crystals when recrystallized from solution. These crystals should be stored in a dry, airtight container to prevent hydrolysis. Some hydrolysis to salicylic acid and acetic acid is unavoidable, however, so acetylsalicylic acid cannot be kept for very long periods of time without loss in purity.
Preparation
Acetylsalicylic acid is prepared via the esterification of salicylic acid with acetic anhydride. This reaction is performed in the presence of a small amount of catalyst, such as sulfuric acid or phosphoric acid. The reaction yields aspirin and acetic acid. The acetic acid is neutralized and the aspirin is extracted.
Projects
- Make sodium acetylsalicylate
- Picric acid synthesis
- Copper(II) acetylsalicylate synthesis
Handling
Safety
Aspirin should not be consumed in large quantities, as it has anticoagulant properties and can cause gastrointestinal bleeding. Lab grade aspirin should never be consumed.
Storage
In a dry place to prevent hydrolysis.
Disposal
Aspirin can be safely poured down the drain on dumped in the trash.