Difference between revisions of "Methyl formate"
(Added image) |
(→Preparation) |
||
| (16 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Chembox |
| − | [[ | + | | Name = Methyl formate |
| + | | Reference = | ||
| + | | IUPACName = Methyl formate | ||
| + | | PIN = Methyl formate | ||
| + | | SystematicName = | ||
| + | | OtherNames = R-611 | ||
| + | <!-- Images --> | ||
| + | | ImageFile = | ||
| + | | ImageSize = | ||
| + | | ImageAlt = | ||
| + | | ImageName = | ||
| + | | ImageFile1 = Methyl formate freshly distilled in RBF.jpg | ||
| + | | ImageSize1 = 280 | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageCaption1 = Freshly distilled pure methyl formate, made from 85% formic acid and methanol. | ||
| + | | ImageFile2 = Methyl formate structure.png | ||
| + | | ImageSize2 = 250 | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageCaption2 = Chemical structure | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = | ||
| + | | ImageSizeL1 = | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| + | | Section1 = {{Chembox Identifiers | ||
| + | | 3DMet = | ||
| + | | Abbreviations = | ||
| + | | SMILES = | ||
| + | }} | ||
| + | | Section2 = {{Chembox Properties | ||
| + | | AtmosphericOHRateConstant = | ||
| + | | Appearance = Colorless volatile liquid | ||
| + | | BoilingPt = | ||
| + | | BoilingPtC = 31-32 | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = | ||
| + | | Density = 0.987 g/cm<sup>3</sup> (15 °C)<br>0.98 g/cm<sup>3</sup> (20 °C) | ||
| + | | Formula = C<sub>2</sub>H<sub>4</sub>O<sub>2</sub> | ||
| + | | HenryConstant = | ||
| + | | LogP = 0.03 | ||
| + | | MolarMass = 60.05 g/mol | ||
| + | | MeltingPt = | ||
| + | | MeltingPtC = −100 | ||
| + | | MeltingPt_ref = | ||
| + | | MeltingPt_notes = | ||
| + | | Odor = Fruity, lemonade-like | ||
| + | | pKa = | ||
| + | | pKb = | ||
| + | | Solubility = 30 g/100 ml (20 °C)<br>23 g/100 ml (25 °C) | ||
| + | | SolubleOther = Miscible with glacial [[acetic acid]], [[acetone]], [[chloroform]], [[ethanol]], [[methanol]] | ||
| + | | Solvent = | ||
| + | | VaporPressure = 476 mmHg (20 °C) | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = | ||
| + | | CrystalStruct = | ||
| + | | MolShape = | ||
| + | }} | ||
| + | | Section4 = {{Chembox Thermochemistry | ||
| + | | DeltaGf = | ||
| + | | DeltaHc = | ||
| + | | DeltaHf = | ||
| + | | Entropy = | ||
| + | | HeatCapacity = | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = | ||
| + | | FrictionSens = | ||
| + | | DetonationV = | ||
| + | | REFactor = | ||
| + | }} | ||
| + | | Section6 = {{Chembox Hazards | ||
| + | | AutoignitionPt = 449 °C (840 °F; 722 K) | ||
| + | | ExploLimits = 4.5%-23% | ||
| + | | ExternalMSDS = [https://www.docdroid.net/GYK0OEu/methyl-formate-sa.pdf.html Simga-Aldrich] | ||
| + | | FlashPt = −19 °C | ||
| + | | LD50 = 1,500 mg/kg (rat, oral)<br>1,622 mg/kg (rabbit, oral) | ||
| + | | LC50 = | ||
| + | | MainHazards = Irritant<br>Flammable<br>Volatile | ||
| + | | NFPA-F = | ||
| + | | NFPA-H = | ||
| + | | NFPA-R = | ||
| + | | NFPA-S = | ||
| + | }} | ||
| + | | Section7 = {{Chembox Related | ||
| + | | OtherAnions = | ||
| + | | OtherCations = | ||
| + | | OtherFunction = | ||
| + | | OtherFunction_label = | ||
| + | | OtherCompounds = [[Ethyl formate]]<br>[[Methyl acetate]] | ||
| + | }} | ||
| + | }} | ||
'''Methyl formate''' or '''methyl methanoate''', is the methyl ester of [[formic acid]], the simplest ester. | '''Methyl formate''' or '''methyl methanoate''', is the methyl ester of [[formic acid]], the simplest ester. | ||
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| − | Methyl formate can be hydrolyzed to [[methanol]] and [[formic acid]]. | + | Methyl formate can be hydrolyzed to [[methanol]] and [[formic acid]] using a strong acid. |
| + | |||
| + | [[Aminolysis]] of methyl formate gives [[formamide]] or [[dimethylformamide]]. | ||
| + | |||
| + | Like its precursor, methanol, methyl formate burns with a nearly invisible flame. | ||
===Physical=== | ===Physical=== | ||
| − | Methyl formate is a colorless organic liquid, with an ethereal odor, low surface tension and high vapor pressure. | + | Methyl formate is a colorless organic liquid, with an ethereal odor, low surface tension and high vapor pressure. It has a melting point of −100 °C and boils at 32 °C. Methyl formate has a density of 0.97 g/cm<sup>3</sup> at standard conditions. It is soluble in water (30 g/100 ml) and other solvents, such as [[acetone]], [[diethyl ether]], [[ethanol]], [[ethyl acetate]]. The flash point of methyl formate is -19 °C and its auto-ignition temperature is 449 °C. |
==Availability== | ==Availability== | ||
| Line 15: | Line 128: | ||
==Preparation== | ==Preparation== | ||
Methyl formate can be synthesized by reacting anhydrous formic acid with dry methanol, over a strong desiccant, such as [[calcium chloride]]. | Methyl formate can be synthesized by reacting anhydrous formic acid with dry methanol, over a strong desiccant, such as [[calcium chloride]]. | ||
| + | |||
| + | : CH<sub>3</sub>OH + HCOOH → HCOOCH<sub>3</sub> + H<sub>2</sub>O | ||
| + | |||
| + | The addition of calcium chloride isn't necessary, but it helps to limit the amount of water being pushed in the condenser by the boiling methyl formate vapors and can also act as boiling chips, which is very important, as due to its low boiling point, methyl formate may bump if the heating element heats the flask too much, unless you have very good temperature control. The resulting distillate is purified by salting out the water and methanol impurities with anh. calcium chloride, followed by drying with anhydrous [[magnesium sulfate|MgSO<sub>4</sub>]]. Due to its low boiling point, methyl formate can be easily purified via fractional distillation. | ||
| + | |||
| + | While a small amount of [[sulfuric acid]] is often added as catalyst during esterification reactions, in this case this isn't necessary, as formic acid is strong enough that it can trigger the reaction on its own, and adding H<sub>2</sub>SO<sub>4</sub> may affect the yield, as formic acid tends to break down in the presence of sulfuric acid to [[carbon monoxide]]. | ||
Industrially, it is prepared by via carbonylation of methanol, using [[sodium methoxide]] as a catalyst and [[pyridine]] as a temperature promoter, in an extremely dry medium. The smallest traces of water can disrupt the reaction. | Industrially, it is prepared by via carbonylation of methanol, using [[sodium methoxide]] as a catalyst and [[pyridine]] as a temperature promoter, in an extremely dry medium. The smallest traces of water can disrupt the reaction. | ||
| − | :CH<sub>3</sub>OH + CO → HCOOCH<sub>3</sub><ref>http://www.bjb.dicp.ac.cn/jngc/2004/2004-04-225.pdf</ref> | + | : CH<sub>3</sub>OH + CO → HCOOCH<sub>3</sub><ref>http://www.bjb.dicp.ac.cn/jngc/2004/2004-04-225.pdf</ref> |
| + | |||
| + | This route however is uneconomical for the amateur chemist, thus the first method is cheaper and more accessible. | ||
==Projects== | ==Projects== | ||
| Line 29: | Line 150: | ||
===Storage=== | ===Storage=== | ||
| − | Due to its low boiling point, | + | Due to its low boiling point, methyl formate should be stored in closed bottles, away from any source of heat or light, preferable in a fridge. This is mandatory during hot summers. |
===Disposal=== | ===Disposal=== | ||
| − | Methyl formate can be safely burned. | + | Methyl formate can be safely burned, outside or in an incinerator, as its gaseous products are only water and carbon dioxide. Like methanol, its flame is nearly invisible, and thus it may pose a safety hazard. To avoid this issue, when burning this compound, it should be mixed with another solvent, like acetone or ethanol, which would turn the flame visible. |
==References== | ==References== | ||
| Line 39: | Line 160: | ||
*[http://www.sciencemadness.org/talk/viewthread.php?tid=9980 methyl formate] | *[http://www.sciencemadness.org/talk/viewthread.php?tid=9980 methyl formate] | ||
*[https://sciencemadness.org/talk/viewthread.php?tid=7289 Chlorination of methyl formate] | *[https://sciencemadness.org/talk/viewthread.php?tid=7289 Chlorination of methyl formate] | ||
| + | |||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
[[Category:Organic compounds]] | [[Category:Organic compounds]] | ||
| + | [[Category:Formates]] | ||
[[Category:Esters]] | [[Category:Esters]] | ||
[[Category:Solvents]] | [[Category:Solvents]] | ||
| + | [[Category:Polar solvents]] | ||
| + | [[Category:Aprotic solvents]] | ||
[[Category:Fragrant compounds]] | [[Category:Fragrant compounds]] | ||
[[Category:Volatile chemicals]] | [[Category:Volatile chemicals]] | ||
| + | [[Category:Materials unstable in basic solution]] | ||
| + | [[Category:Liquids]] | ||
Latest revision as of 09:11, 23 June 2024
 Freshly distilled pure methyl formate, made from 85% formic acid and methanol.
| |
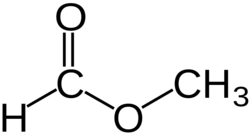 Chemical structure
| |
| Names | |
|---|---|
| IUPAC name
Methyl formate
| |
| Preferred IUPAC name
Methyl formate | |
| Other names
R-611
| |
| Properties | |
| C2H4O2 | |
| Molar mass | 60.05 g/mol |
| Appearance | Colorless volatile liquid |
| Odor | Fruity, lemonade-like |
| Density | 0.987 g/cm3 (15 °C) 0.98 g/cm3 (20 °C) |
| Melting point | −100 °C (−148 °F; 173 K) |
| Boiling point | 31–32 °C (88–90 °F; 304–305 K) |
| 30 g/100 ml (20 °C) 23 g/100 ml (25 °C) | |
| Solubility | Miscible with glacial acetic acid, acetone, chloroform, ethanol, methanol |
| Vapor pressure | 476 mmHg (20 °C) |
| Hazards | |
| Safety data sheet | Simga-Aldrich |
| Flash point | −19 °C |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
1,500 mg/kg (rat, oral) 1,622 mg/kg (rabbit, oral) |
| Related compounds | |
| Related compounds
|
Ethyl formate Methyl acetate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methyl formate or methyl methanoate, is the methyl ester of formic acid, the simplest ester.
Contents
[hide]Properties
Chemical
Methyl formate can be hydrolyzed to methanol and formic acid using a strong acid.
Aminolysis of methyl formate gives formamide or dimethylformamide.
Like its precursor, methanol, methyl formate burns with a nearly invisible flame.
Physical
Methyl formate is a colorless organic liquid, with an ethereal odor, low surface tension and high vapor pressure. It has a melting point of −100 °C and boils at 32 °C. Methyl formate has a density of 0.97 g/cm3 at standard conditions. It is soluble in water (30 g/100 ml) and other solvents, such as acetone, diethyl ether, ethanol, ethyl acetate. The flash point of methyl formate is -19 °C and its auto-ignition temperature is 449 °C.
Availability
Methyl formate is available from chemical suppliers.
Preparation
Methyl formate can be synthesized by reacting anhydrous formic acid with dry methanol, over a strong desiccant, such as calcium chloride.
- CH3OH + HCOOH → HCOOCH3 + H2O
The addition of calcium chloride isn't necessary, but it helps to limit the amount of water being pushed in the condenser by the boiling methyl formate vapors and can also act as boiling chips, which is very important, as due to its low boiling point, methyl formate may bump if the heating element heats the flask too much, unless you have very good temperature control. The resulting distillate is purified by salting out the water and methanol impurities with anh. calcium chloride, followed by drying with anhydrous MgSO4. Due to its low boiling point, methyl formate can be easily purified via fractional distillation.
While a small amount of sulfuric acid is often added as catalyst during esterification reactions, in this case this isn't necessary, as formic acid is strong enough that it can trigger the reaction on its own, and adding H2SO4 may affect the yield, as formic acid tends to break down in the presence of sulfuric acid to carbon monoxide.
Industrially, it is prepared by via carbonylation of methanol, using sodium methoxide as a catalyst and pyridine as a temperature promoter, in an extremely dry medium. The smallest traces of water can disrupt the reaction.
- CH3OH + CO → HCOOCH3[1]
This route however is uneconomical for the amateur chemist, thus the first method is cheaper and more accessible.
Projects
- Formamide synthesis
- Organic extractions
Handling
Safety
Methyl formate vapors may irritate lungs, so it's best to work in a well ventilated area. Since its boiling point is lower than the human body temperature, samples of methyl formate should not be kept too long in hand.
Storage
Due to its low boiling point, methyl formate should be stored in closed bottles, away from any source of heat or light, preferable in a fridge. This is mandatory during hot summers.
Disposal
Methyl formate can be safely burned, outside or in an incinerator, as its gaseous products are only water and carbon dioxide. Like methanol, its flame is nearly invisible, and thus it may pose a safety hazard. To avoid this issue, when burning this compound, it should be mixed with another solvent, like acetone or ethanol, which would turn the flame visible.