Difference between revisions of "2-Nitrotoluene"
| (5 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
{{Chembox | {{Chembox | ||
| − | | Name =2- | + | | Name =2-Nitrotoluene |
| Reference = | | Reference = | ||
| − | | IUPACName =1- | + | | IUPACName = 1-Methyl-2-nitro-benzene |
| PIN = | | PIN = | ||
| − | | SystematicName =1- | + | | SystematicName = 1-Methyl-2-nitro-benzene |
| − | | OtherNames = o- | + | | OtherNames = o-Nitrotoluene |
<!-- Images --> | <!-- Images --> | ||
| − | | ImageFile = 2-nitrotoluene_structure.png | + | | ImageFile = 2-nitrotoluene_structure-2.png |
| ImageSize = | | ImageSize = | ||
| ImageAlt = | | ImageAlt = | ||
| Line 106: | Line 105: | ||
| OtherFunction = | | OtherFunction = | ||
| OtherFunction_label = | | OtherFunction_label = | ||
| − | | OtherCompounds = 3-nitrotoluene<br>4-nitrotoluene<br>[[Trinitrotoluene]] | + | | OtherCompounds = [[3-nitrotoluene]]<br>[[4-nitrotoluene]]<br>[[Trinitrotoluene]] |
}} | }} | ||
}} | }} | ||
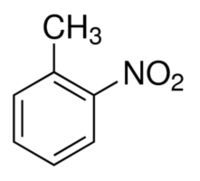
| − | '''2- | + | '''2-Nitrotoluene''', also known as '''o-nitrotoluene''', is an isomer of mononitrotoluene. It is a yellow, oily liquid that is slightly more dense than water. |
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| − | Through many different methods, 2-nitrotoluene is oxidized to [[2-nitrobenzaldehyde]] which is a direct precursor to [[indigo]] | + | Through many different methods, 2-nitrotoluene is oxidized to [[2-nitrobenzaldehyde]] which is a direct precursor to [[indigo dye]].<br> |
The nitro group on 2-nitrotoluene may be reduced to yield [[2-methylaniline]] (2-toluidine). | The nitro group on 2-nitrotoluene may be reduced to yield [[2-methylaniline]] (2-toluidine). | ||
===Physical=== | ===Physical=== | ||
| − | 2- | + | 2-Nitrotoluene is a yellow, oily liquid. It has a strong odor resembling almonds, typical of nitrated aromatic compounds. |
==Availability== | ==Availability== | ||
| Line 123: | Line 122: | ||
==Preparation== | ==Preparation== | ||
| − | A mixture of mononitrotoluene isomers can be prepared from the [[nitration]] of [[toluene]] between - | + | A mixture of mononitrotoluene isomers can be prepared from the [[nitration]] of [[toluene]] between -10 ºC and 30 ºC. Lower temperatures result in little or no reaction, while higher temperatures will result in double nitration forming dinitrotoluenes. <br> |
'''Relevant thread:''' [https://www.sciencemadness.org/whisper/viewthread.php?tid=29111 Preparation of Mononitrotoluenes (o-, p-)] | '''Relevant thread:''' [https://www.sciencemadness.org/whisper/viewthread.php?tid=29111 Preparation of Mononitrotoluenes (o-, p-)] | ||
==Projects== | ==Projects== | ||
| − | *Make indigo | + | *Make [[indigo dye]] |
*Make di- and trinitrotoluene | *Make di- and trinitrotoluene | ||
==Handling== | ==Handling== | ||
===Safety=== | ===Safety=== | ||
| − | 2- | + | 2-Nitrotoluene is quite toxic and a suspected carcinogen. It must be handled with care, and should be used only in a fume hood. Care should be taken to avoid inhaling its vapors. |
===Storage=== | ===Storage=== | ||
| Line 138: | Line 137: | ||
===Disposal=== | ===Disposal=== | ||
| − | 2-Nitrotoluene can be destroyed by burning it. This must be done outside, as burning it will give off soot, carbon monoxide and other | + | 2-Nitrotoluene can be destroyed by burning it. This must be done outside, as burning it will give off soot, carbon monoxide and other harmful fumes. |
| − | 2- | + | 2-Nitrotoluene can be safely destroyed with Fenton's reagent. However, it is best to add very small amounts of the compound, preferably dilute, as the destruction is exothermic and may aerosolize other aromatic compounds created from the incomplete Fenton oxidation. Using a [[Ultraviolet lamp|UV lamp]] will improve the performance of the neutralization process. |
==References== | ==References== | ||
| − | [https://www.fishersci.com/shop/msdsproxy?productName=AC129030010&productDescription=2-NITROTOLUENE%2C+99%2B%25+1LT&catNo=AC12903-0010&vendorId=VN00032119&storeId=10652 MSDS] | + | *[https://www.fishersci.com/shop/msdsproxy?productName=AC129030010&productDescription=2-NITROTOLUENE%2C+99%2B%25+1LT&catNo=AC12903-0010&vendorId=VN00032119&storeId=10652 MSDS] |
<references/> | <references/> | ||
===Relevant Sciencemadness threads=== | ===Relevant Sciencemadness threads=== | ||
| Line 151: | Line 150: | ||
[[Category:Organic compounds]] | [[Category:Organic compounds]] | ||
[[Category:Aromatic compounds]] | [[Category:Aromatic compounds]] | ||
| − | [[Category: | + | [[Category:Nitro compounds]] |
| − | [[Category: | + | [[Category:Nitroaromatics]] |
[[Category:Liquids]] | [[Category:Liquids]] | ||
| + | [[Category:Carcinogenic]] | ||
Latest revision as of 15:45, 28 December 2023
| |
| Names | |
|---|---|
| IUPAC name
1-Methyl-2-nitro-benzene
| |
| Systematic IUPAC name
1-Methyl-2-nitro-benzene | |
| Other names
o-Nitrotoluene
| |
| Properties | |
| C6H4CH3NO2 | |
| Molar mass | 137.14 |
| Appearance | Yellow liquid |
| Odor | Weak, aromatic |
| Density | 1.161 g/cm3 (20 °C) |
| Melting point | −10.4 °C (13.3 °F; 262.8 K) |
| Boiling point | 222 °C (432 °F; 495 K) |
| 0.0609 g/100 ml (20 °C) 0.065 m/100 ml (30 °C) | |
| Solubility | Miscible with benzene, carbon tetrachloride, chloroform, diethyl ether, ethanol, petroleum ether |
| Vapor pressure | 0.1 mmHg (20 °C) |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 106 °C (223 °F; 379 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
891 mg/kg (rat, oral) 970 mg/kg (mouse, oral) 1,750 mg/kg (rabbit, oral) |
| Related compounds | |
| Related compounds
|
3-nitrotoluene 4-nitrotoluene Trinitrotoluene |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-Nitrotoluene, also known as o-nitrotoluene, is an isomer of mononitrotoluene. It is a yellow, oily liquid that is slightly more dense than water.
Contents
Properties
Chemical
Through many different methods, 2-nitrotoluene is oxidized to 2-nitrobenzaldehyde which is a direct precursor to indigo dye.
The nitro group on 2-nitrotoluene may be reduced to yield 2-methylaniline (2-toluidine).
Physical
2-Nitrotoluene is a yellow, oily liquid. It has a strong odor resembling almonds, typical of nitrated aromatic compounds.
Availability
Nitrotoluenes are not available in any consumer products due to being toxic and possibly carcinogenic, and are not found in nature.
Preparation
A mixture of mononitrotoluene isomers can be prepared from the nitration of toluene between -10 ºC and 30 ºC. Lower temperatures result in little or no reaction, while higher temperatures will result in double nitration forming dinitrotoluenes.
Relevant thread: Preparation of Mononitrotoluenes (o-, p-)
Projects
- Make indigo dye
- Make di- and trinitrotoluene
Handling
Safety
2-Nitrotoluene is quite toxic and a suspected carcinogen. It must be handled with care, and should be used only in a fume hood. Care should be taken to avoid inhaling its vapors.
Storage
A glass bottle with a tight fitting, chemical resistant cap is sufficient for containing 2-nitrotoluene. For better safety, store the bottle inside of another larger container to limit exposure.
Disposal
2-Nitrotoluene can be destroyed by burning it. This must be done outside, as burning it will give off soot, carbon monoxide and other harmful fumes.
2-Nitrotoluene can be safely destroyed with Fenton's reagent. However, it is best to add very small amounts of the compound, preferably dilute, as the destruction is exothermic and may aerosolize other aromatic compounds created from the incomplete Fenton oxidation. Using a UV lamp will improve the performance of the neutralization process.