Difference between revisions of "Copper(I) chloride"
| (10 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | '''Copper(I) chloride''', | + | {{Chembox |
| + | | Name = Copper(I) chloride | ||
| + | | Reference = | ||
| + | | IUPACName = Copper(I) chloride | ||
| + | | PIN = | ||
| + | | SystematicName = | ||
| + | | OtherNames = Copper monochloride<br>Cuprous chloride | ||
| + | <!-- Images --> | ||
| + | | ImageFile = Copper(I) chloride SA by Ormarion.jpg | ||
| + | | ImageSize = 250px | ||
| + | | ImageCaption = Sealed vial containing CuCl | ||
| + | | ImageAlt = | ||
| + | | ImageName = | ||
| + | | ImageFile1 = | ||
| + | | ImageSize1 = | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageFile2 = | ||
| + | | ImageSize2 = | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = | ||
| + | | ImageSizeL1 = | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| + | | Section1 = {{Chembox Identifiers | ||
| + | | 3DMet = | ||
| + | | Abbreviations = | ||
| + | | SMILES = | ||
| + | }} | ||
| + | | Section2 = {{Chembox Properties | ||
| + | | AtmosphericOHRateConstant = | ||
| + | | Appearance = White solid | ||
| + | | BoilingPt = | ||
| + | | BoilingPtC = 1,490 | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = (decomposes) | ||
| + | | Density = 4.14 g/cm<sup>3</sup> | ||
| + | | Formula = CuCl | ||
| + | | HenryConstant = | ||
| + | | LogP = | ||
| + | | MolarMass = 98.999 g/mol | ||
| + | | MeltingPt = | ||
| + | | MeltingPtC = 423 | ||
| + | | MeltingPt_ref = | ||
| + | | MeltingPt_notes = | ||
| + | | pKa = | ||
| + | | pKb = | ||
| + | | Solubility = 0.47 g/100 ml (20 °C) | ||
| + | | SolubleOther = Soluble in aq. [[ammonia]], conc. [[Hydrochloric acid|HCl]]<br>Insoluble in [[acetone]], [[ethanol]] | ||
| + | | Solvent = | ||
| + | | VaporPressure = ~0 mmHg | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = | ||
| + | | CrystalStruct = Zincblende, cF20 | ||
| + | | MolShape = | ||
| + | }} | ||
| + | | Section4 = {{Chembox Thermochemistry | ||
| + | | DeltaGf = | ||
| + | | DeltaHc = | ||
| + | | DeltaHf = | ||
| + | | Entropy = | ||
| + | | HeatCapacity = | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = | ||
| + | | FrictionSens = | ||
| + | | DetonationV = | ||
| + | | REFactor = | ||
| + | }} | ||
| + | | Section6 = {{Chembox Hazards | ||
| + | | AutoignitionPt = Non-flammable | ||
| + | | ExploLimits = | ||
| + | | ExternalMSDS = [https://www.docdroid.net/9LYmcfy/copperi-chloride-sa.pdf.html Sigma-Aldrich] | ||
| + | | FlashPt = Non-flammable | ||
| + | | LD50 = 140 mg/kg | ||
| + | | LC50 = | ||
| + | | MainHazards = Harmful | ||
| + | | NFPA-F = | ||
| + | | NFPA-H = | ||
| + | | NFPA-R = | ||
| + | | NFPA-S = | ||
| + | }} | ||
| + | | Section7 = {{Chembox Related | ||
| + | | OtherAnions = | ||
| + | | OtherCations = | ||
| + | | OtherFunction = | ||
| + | | OtherFunction_label = | ||
| + | | OtherCompounds = [[Copper(II) chloride]] | ||
| + | }} | ||
| + | }} | ||
| + | '''Copper(I) chloride''', also called '''cuprous chloride''', is an inorganic chemical compound, with the chemical formula '''CuCl'''. It is a white, almost insoluble salt which is slowly oxidized by air to Cu(II). | ||
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| − | CuCl is almost completely insoluble in water. It does however form complexes and dissolve in concentrated [[hydrochloric acid]] and | + | CuCl is almost completely insoluble in water. It does however form complexes and dissolve in concentrated [[hydrochloric acid]] and ammonium hydroxide (aq. [[ammonia]]), as well as in cyanide and thiosulfate solutions. |
===Physical=== | ===Physical=== | ||
| − | Pure samples of copper(I) chloride appear as white, dense, cubical crystals. As it is slowly oxidized in air, older samples may appear dirty green or brown. | + | Pure samples of copper(I) chloride appear as white, dense, cubical crystals. As it is slowly oxidized in air, older samples may appear dirty green or brown.<ref>Copper(I) chloride, Wikipedia (http://en.wikipedia.org/wiki/Copper%28I%29_chloride)</ref> |
==Preparation== | ==Preparation== | ||
| − | Copper(I) chloride can be prepared by reduction of copper(II) ions in presence of chloride ions | + | Copper(I) chloride can be prepared by reduction of copper(II) ions in presence of chloride ions. |
| − | + | ||
| − | + | : CuCl<sub>2</sub> → CuCl + Cl<sup>-<sup> | |
| − | + | ||
| − | The primary explosive [[copper(I) acetylide]] is made by passing [[acetylene | + | Possible methods include bubbling [[sulfur dioxide]] through an aqueous solution of [[copper(II) chloride]], or heating a solution of copper sulfate, sodium chloride and [[ascorbic acid]]. |
| + | |||
| + | : CuCl<sub>2</sub> + SO<sub>2</sub> + 2 H<sub>2</sub>O → 2 CuCl + H<sub>2</sub>SO<sub>4</sub> + 2 HCl | ||
| + | :2 CuCl<sub>2</sub> + C<sub>6</sub>H<sub>8</sub>O<sub>6</sub> → 2 CuCl + 2HCl + C<sub>6</sub>H<sub>6</sub>O<sub>6</sub> | ||
| + | |||
| + | It can also be produced by boiling copper(II) chloride and copper metal in hydrochloric acid. | ||
| + | |||
| + | : CuCl<sub>2</sub> + Cu → 2 CuCl | ||
| + | |||
| + | Originally, CuCl was first made by reducing [[mercury(II) chloride]] with copper metal:<ref>https://quod.lib.umich.edu/e/eebo/A29017.0001.001?rgn=main;view=fulltext</ref> | ||
| + | |||
| + | : HgCl<sub>2</sub> + 2 Cu → 2 CuCl + Hg | ||
| + | |||
| + | Industrially it is made by direct combination of copper metal and chlorine at 450–900 °C: | ||
| + | |||
| + | :2 Cu + Cl<sub>2</sub> → 2 CuCl | ||
| + | |||
| + | ==Projects== | ||
| + | *Copper(I) chloride can be used to make [[copper oxychloride]] by oxidation in air. | ||
| + | *The primary explosive [[copper(I) acetylide]] is made by passing [[acetylene]] gas through a solution of CuCl in aqueous ammonia. | ||
| + | *Purify [[mercury(II) fulminate]] | ||
==Handling== | ==Handling== | ||
===Safety=== | ===Safety=== | ||
| − | Cuprous chloride is irritant and corrosive to eyes and skin. Protection clothing should be worn when handling it. | + | Cuprous chloride is irritant and corrosive to eyes and skin. Protection clothing should be worn when handling it. |
===Storage=== | ===Storage=== | ||
| + | CuCl should be kept in sealed containers, away from oxygen. Schlenk flasks are a good storage container. | ||
===Disposal=== | ===Disposal=== | ||
| + | CuCl can be oxidized with oxygen or hydrogen peroxide to the more soluble CuCl<sub>2</sub>, which can be reduced to metallic copper with a more reactive metal, such as iron or zinc. | ||
| + | |||
| + | ==Gallery== | ||
| + | <gallery widths="220" position="center" columns="4" orientation="none"> | ||
| + | Copper_I_chloride_exf_community.jpg|CuCl prepared by a member of the Ex&F community. | ||
| + | </gallery> | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| − | |||
===Relevant Sciencemadness threads=== | ===Relevant Sciencemadness threads=== | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=61615 Preparation Of CuCl And Problems Drying] | ||
| + | [[Category:Chemical compounds]] | ||
| + | [[Category:Inorganic compounds]] | ||
[[Category:Copper compounds]] | [[Category:Copper compounds]] | ||
[[Category:Chlorides]] | [[Category:Chlorides]] | ||
| − | |||
| − | |||
[[Category:Common catalysts]] | [[Category:Common catalysts]] | ||
| + | [[Category:Halides]] | ||
| + | [[Category:Air-sensitive materials]] | ||
Latest revision as of 08:17, 23 September 2023
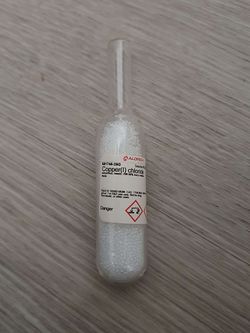 Sealed vial containing CuCl
| |
| Names | |
|---|---|
| IUPAC name
Copper(I) chloride
| |
| Other names
Copper monochloride
Cuprous chloride | |
| Properties | |
| CuCl | |
| Molar mass | 98.999 g/mol |
| Appearance | White solid |
| Density | 4.14 g/cm3 |
| Melting point | 423 °C (793 °F; 696 K) |
| Boiling point | 1,490 °C (2,710 °F; 1,760 K) (decomposes) |
| 0.47 g/100 ml (20 °C) | |
| Solubility | Soluble in aq. ammonia, conc. HCl Insoluble in acetone, ethanol |
| Vapor pressure | ~0 mmHg |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
140 mg/kg |
| Related compounds | |
| Related compounds
|
Copper(II) chloride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Copper(I) chloride, also called cuprous chloride, is an inorganic chemical compound, with the chemical formula CuCl. It is a white, almost insoluble salt which is slowly oxidized by air to Cu(II).
Contents
Properties
Chemical
CuCl is almost completely insoluble in water. It does however form complexes and dissolve in concentrated hydrochloric acid and ammonium hydroxide (aq. ammonia), as well as in cyanide and thiosulfate solutions.
Physical
Pure samples of copper(I) chloride appear as white, dense, cubical crystals. As it is slowly oxidized in air, older samples may appear dirty green or brown.[1]
Preparation
Copper(I) chloride can be prepared by reduction of copper(II) ions in presence of chloride ions.
- CuCl2 → CuCl + Cl-
Possible methods include bubbling sulfur dioxide through an aqueous solution of copper(II) chloride, or heating a solution of copper sulfate, sodium chloride and ascorbic acid.
- CuCl2 + SO2 + 2 H2O → 2 CuCl + H2SO4 + 2 HCl
- 2 CuCl2 + C6H8O6 → 2 CuCl + 2HCl + C6H6O6
It can also be produced by boiling copper(II) chloride and copper metal in hydrochloric acid.
- CuCl2 + Cu → 2 CuCl
Originally, CuCl was first made by reducing mercury(II) chloride with copper metal:[2]
- HgCl2 + 2 Cu → 2 CuCl + Hg
Industrially it is made by direct combination of copper metal and chlorine at 450–900 °C:
- 2 Cu + Cl2 → 2 CuCl
Projects
- Copper(I) chloride can be used to make copper oxychloride by oxidation in air.
- The primary explosive copper(I) acetylide is made by passing acetylene gas through a solution of CuCl in aqueous ammonia.
- Purify mercury(II) fulminate
Handling
Safety
Cuprous chloride is irritant and corrosive to eyes and skin. Protection clothing should be worn when handling it.
Storage
CuCl should be kept in sealed containers, away from oxygen. Schlenk flasks are a good storage container.
Disposal
CuCl can be oxidized with oxygen or hydrogen peroxide to the more soluble CuCl2, which can be reduced to metallic copper with a more reactive metal, such as iron or zinc.
Gallery
References
- ↑ Copper(I) chloride, Wikipedia (http://en.wikipedia.org/wiki/Copper%28I%29_chloride)
- ↑ https://quod.lib.umich.edu/e/eebo/A29017.0001.001?rgn=main;view=fulltext
Relevant Sciencemadness threads
