Difference between revisions of "Benzododecinium bromide"
From Sciencemadness Wiki
| (2 intermediate revisions by the same user not shown) | |||
| Line 57: | Line 57: | ||
| MeltingPt_ref = | | MeltingPt_ref = | ||
| MeltingPt_notes = | | MeltingPt_notes = | ||
| + | | Odor = Odorless | ||
| pKa = | | pKa = | ||
| pKb = | | pKb = | ||
| Line 115: | Line 116: | ||
==Preparation== | ==Preparation== | ||
| − | + | Preparation for this compound can be found in literature. | |
==Projects== | ==Projects== | ||
| Line 130: | Line 131: | ||
===Disposal=== | ===Disposal=== | ||
Should be diluted strongly before disposal. | Should be diluted strongly before disposal. | ||
| + | |||
| + | ==Gallery== | ||
| + | <gallery widths="220" position="center" columns="4" orientation="none"> | ||
| + | 2013-06-29-csa12533.jpg|Benzododecinium bromide as 1% solution. | ||
| + | </gallery> | ||
==References== | ==References== | ||
Latest revision as of 22:45, 26 August 2023
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
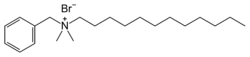 Skeletal structure of benzododecium bromide.
| |
| Names | |
|---|---|
| IUPAC name
Benzyl-dodecyl-dimethylammonium bromide
| |
| Other names
Ajatin
Bacfor BL Benzalkonium bromide | |
| Properties | |
| C21H38NBr | |
| Molar mass | 384.437 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Melting point | 46–48 °C (115–118 °F; 319–321 K) |
| Boiling point | Decomposes |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 110 °C (230 °F; 383 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
230 mg/kg (rat, oral) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Benzododecinium bromide is an antiseptic and disinfectant produced under trade mark Ajatin.
Contents
Properties
Chemical
Physical
Benzododecinium bromide is a white solid.
Availability
Benzododecium bromide can be bought as a disinfectant (water solution), altough the concentration is quite low.
Preparation
Preparation for this compound can be found in literature.
Projects
- Desinfectant
- Absorbent for hexavalent chromium[1]
Handling
Safety
Little data exists about its toxicity.
Storage
In closed bottles.
Disposal
Should be diluted strongly before disposal.