Difference between revisions of "Potassium hydroxide"
| (23 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | {{Chembox | |
| − | [[ | + | | Name = Potassium hydroxide |
| − | '''Potassium hydroxide''' is a white solid with the formula [[Potassium|K]][[Hydroxide|OH]]. It finds many applications in the lab as a general base, though [[sodium hydroxide]] is generally preferred as it is easier to find and slightly cheaper. | + | | Reference = |
| + | | IUPACName = Potassium hydroxide | ||
| + | | PIN = | ||
| + | | SystematicName = Potassium hydroxide | ||
| + | | OtherNames = Caustic potash<br>Lye<br>Potash lye<br>Potassia<br>Potassium hydrate | ||
| + | <!-- Images --> | ||
| + | | ImageFile = File:Potassium hydroxide.jpg | ||
| + | | ImageSize = | ||
| + | | ImageAlt = | ||
| + | | ImageName = | ||
| + | | ImageCaption = Potassium hydroxide flakes | ||
| + | | ImageFile1 = KOH.png | ||
| + | | ImageSize1 = | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageFile2 = | ||
| + | | ImageSize2 = | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = | ||
| + | | ImageSizeL1 = | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| + | | Section1 = {{Chembox Identifiers | ||
| + | | 3DMet = | ||
| + | | Abbreviations = | ||
| + | | SMILES = | ||
| + | }} | ||
| + | | Section2 = {{Chembox Properties | ||
| + | | AtmosphericOHRateConstant = | ||
| + | | Appearance = White solid, [[deliquescent]] | ||
| + | | BoilingPt = | ||
| + | | BoilingPtC = 1327 | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = | ||
| + | | Density = 2.044 g/cm<sup>3</sup> (20 °C)<br>2.12 g/cm<sup>3</sup> (25 °C) | ||
| + | | Formula = KOH | ||
| + | | HenryConstant = | ||
| + | | LogP = | ||
| + | | MolarMass = 56.106 g/mol | ||
| + | | MeltingPt = | ||
| + | | MeltingPtC = 360-380 | ||
| + | | MeltingPt_ref = | ||
| + | | MeltingPt_notes = | ||
| + | | Odor = Odorless | ||
| + | | pKa = | ||
| + | | pKb = | ||
| + | | Solubility = 85 g/100 ml (-23.2 °C)<br>97 g/100 ml (0 °C)<br>121 g/100 ml (25 °C)<br>138.3 g/100 ml (50 °C)<br> 162.9 g/100 ml (100 °C) | ||
| + | | SolubleOther = Soluble in [[ethanol]], [[glycerol]], [[isopropanol]], [[methanol]]<br>Insoluble in anh. [[ammonia]], [[diethyl ether]] | ||
| + | | Solubility1 = 14 g/100 g (28 °C) | ||
| + | | Solvent1 = isopropanol | ||
| + | | Solubility2 = 55 g/100 g (28 °C) | ||
| + | | Solvent2 = methanol | ||
| + | | VaporPressure = ~0 mmHg | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = | ||
| + | | CrystalStruct = | ||
| + | | MolShape = | ||
| + | }} | ||
| + | | Section4 = {{Chembox Thermochemistry | ||
| + | | DeltaGf = -380.2 kJ/mol | ||
| + | | DeltaHc = | ||
| + | | DeltaHf = -425.8 kJ/mol | ||
| + | | Entropy = 79.32 J·mol<sup>-1</sup>·K<sup>-1</sup> | ||
| + | | HeatCapacity = 65.87 J·mol<sup>-1</sup>·K<sup>-1</sup> | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = | ||
| + | | FrictionSens = | ||
| + | | DetonationV = | ||
| + | | REFactor = | ||
| + | }} | ||
| + | | Section6 = {{Chembox Hazards | ||
| + | | AutoignitionPt = Non-flammable | ||
| + | | ExploLimits = | ||
| + | | ExternalMSDS = [https://www.docdroid.net/ritEQcd/potassium-hydroxide-sa.pdf.html Sigma-Aldrich] | ||
| + | | FlashPt = Non-flammable | ||
| + | | LD50 = 273 mg/kg (rat, oral) | ||
| + | | LC50 = | ||
| + | | MainHazards = Corrosive | ||
| + | | NFPA-F = | ||
| + | | NFPA-H = | ||
| + | | NFPA-R = | ||
| + | | NFPA-S = | ||
| + | }} | ||
| + | | Section7 = {{Chembox Related | ||
| + | | OtherAnions = | ||
| + | | OtherCations = | ||
| + | | OtherFunction = [[Lithium hydroxide]]<br>[[Sodium hydroxide]]<br>[[Rubidium hydroxide]]<br>[[Caesium hydroxide]] | ||
| + | | OtherFunction_label = [[:Category:Alkalis|alkali hydroxides]] | ||
| + | | OtherCompounds = | ||
| + | }} | ||
| + | }} | ||
| + | '''Potassium hydroxide''' is a white solid with the formula '''[[Potassium|K]][[Hydroxide|OH]]'''. It finds many applications in the lab as a general base, though [[sodium hydroxide]] is generally preferred as it is easier to find and slightly cheaper. | ||
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| − | Potassium hydroxide dissolves in water and fully dissociates to give a solution of potassium ions and hydroxide ions. Potassium hydroxide solutions will very readily absorb atmospheric carbon dioxide to form [[potassium carbonate]], and must be protected from the atmosphere. Being a strong base, potassium hydroxide also has the ability to dissolve glass (particularly so at higher temperatures) so solution must be kept in a chemically resistant plastic such as [[polyethylene|high density polyethylene (HDPE) | + | Potassium hydroxide dissolves in water and fully dissociates to give a solution of potassium ions and hydroxide ions. Potassium hydroxide solutions will very readily absorb atmospheric carbon dioxide to form [[potassium carbonate]], and must be protected from the atmosphere. Being a strong base, potassium hydroxide also has the ability to dissolve glass (particularly so at higher temperatures) so solution must be kept in a chemically resistant plastic containers, such as [[polyethylene|high density polyethylene]] (HDPE). |
Potassium hydroxide can be reacted with the corresponding acids to yield potassium salts. Potassium hydroxide is used along with [[manganese dioxide]] and an oxidizer to form crude [[potassium manganate]] at very high temperatures. | Potassium hydroxide can be reacted with the corresponding acids to yield potassium salts. Potassium hydroxide is used along with [[manganese dioxide]] and an oxidizer to form crude [[potassium manganate]] at very high temperatures. | ||
| Line 17: | Line 128: | ||
Potassium hydroxide is available reasonably cheaply from soap making suppliers and biodiesel companies such as [[Lab_suppliers#Duda_Diesel|Duda Diesel]]. It is also available from [[Lab_suppliers#Elemental_Scientific.2C_LLC|Elemental Scientific]] at a slightly higher cost. | Potassium hydroxide is available reasonably cheaply from soap making suppliers and biodiesel companies such as [[Lab_suppliers#Duda_Diesel|Duda Diesel]]. It is also available from [[Lab_suppliers#Elemental_Scientific.2C_LLC|Elemental Scientific]] at a slightly higher cost. | ||
| − | Certain barbecue cleaning products contain 25% KOH. | + | Certain barbecue cleaning products contain 25% KOH, as aqueous solution. |
==Preparation== | ==Preparation== | ||
| − | Preparation of solid potassium hydroxide is difficult in an amateur setting due to the highly hygroscopic nature of potassium hydroxide. | + | Preparation of solid potassium hydroxide is difficult in an amateur setting due to the highly hygroscopic nature of potassium hydroxide. |
| + | |||
| + | Historically, potassium hydroxide was made by reacting [[potassium carbonate]] (potash) with [[calcium hydroxide]] (slaked lime). | ||
| + | |||
| + | : Ca(OH)<sub>2</sub> + K<sub>2</sub>CO<sub>3</sub> → CaCO<sub>3</sub> + 2 KOH | ||
| + | |||
| + | This method can be performed by the amateur chemist, though it requires corrosion resistant containers when drying or concentrating the potassium hydroxide. Another inconvenient represents the organic residues from the wood ash, which are difficult to remove. They can however be removed completely by roasting the potash in the kiln at very high temperatures in an excess of oxygen. | ||
| + | |||
| + | Heating potassium carbonate at over 891 °C will yield potassium oxide that will react quickly and extremely exothermic with water to form hydroxide. It will also react with any carbon dioxide immediately at lower temperatures. This method is not very practical, as it requires high temperatures. | ||
| + | |||
| + | Electrolysis of a saturated solution of KCl with an ion exchange membrane will also yield potassium hydroxide. This preparation method is similar to that for [[sodium hydroxide]]. | ||
| + | |||
| + | Another route, though expensive, involves the reaction of [[potassium]] metal or potassium oxide with water. | ||
==Projects== | ==Projects== | ||
* Make [[potassium permanganate]] solution | * Make [[potassium permanganate]] solution | ||
| + | * Make potassium manganate solid | ||
* Produce clean [[potassium]] metal | * Produce clean [[potassium]] metal | ||
* Make liquid soap | * Make liquid soap | ||
| + | * Make an alkali battery | ||
| + | * Identify certain species of fungi | ||
| + | * Synthesize [[potassium ferrate]] | ||
==Handling== | ==Handling== | ||
| Line 36: | Line 163: | ||
===Disposal=== | ===Disposal=== | ||
Potassium hydroxide will turn to potassium carbonate if left in open air, which poses no toxicity to the environment and it's even used as a source of potassium for plants. | Potassium hydroxide will turn to potassium carbonate if left in open air, which poses no toxicity to the environment and it's even used as a source of potassium for plants. | ||
| + | |||
| + | ==Gallery== | ||
| + | <gallery widths="220" position="center" columns="4" orientation="none"> | ||
| + | Potassium hydroxide wiki.jpg|Potassium hydroxide granules. | ||
| + | </gallery> | ||
==References== | ==References== | ||
<references/> | <references/> | ||
===Relevant Sciencemadness threads=== | ===Relevant Sciencemadness threads=== | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=14450 Making KOH from wood ashes why is it yellow ?] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=16623 Storage of KOH solution] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=157830 KOH purification from bbq cleaner] | ||
| + | [[Category:Chemical compounds]] | ||
| + | [[Category:Inorganic compounds]] | ||
| + | [[Category:Potassium compounds]] | ||
| + | [[Category:Bases]] | ||
[[Category:Alkalis]] | [[Category:Alkalis]] | ||
[[Category:Hydroxides]] | [[Category:Hydroxides]] | ||
[[Category:Corrosive chemicals]] | [[Category:Corrosive chemicals]] | ||
[[Category:Chemicals that attack glassware]] | [[Category:Chemicals that attack glassware]] | ||
| + | [[Category:Hygroscopic compounds]] | ||
| + | [[Category:Readily available chemicals]] | ||
| + | [[Category:Essential reagents]] | ||
| + | [[Category:Irritants]] | ||
| + | [[Category:Air-sensitive materials]] | ||
| + | [[Category:Materials unstable in acidic solution]] | ||
Latest revision as of 16:52, 17 August 2023
 Potassium hydroxide flakes
| |
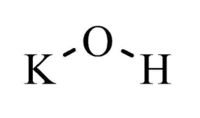
| |
| Names | |
|---|---|
| IUPAC name
Potassium hydroxide
| |
| Systematic IUPAC name
Potassium hydroxide | |
| Other names
Caustic potash
Lye Potash lye Potassia Potassium hydrate | |
| Properties | |
| KOH | |
| Molar mass | 56.106 g/mol |
| Appearance | White solid, deliquescent |
| Odor | Odorless |
| Density | 2.044 g/cm3 (20 °C) 2.12 g/cm3 (25 °C) |
| Melting point | 360–380 °C (680–716 °F; 633–653 K) |
| Boiling point | 1,327 °C (2,421 °F; 1,600 K) |
| 85 g/100 ml (-23.2 °C) 97 g/100 ml (0 °C) 121 g/100 ml (25 °C) 138.3 g/100 ml (50 °C) 162.9 g/100 ml (100 °C) | |
| Solubility | Soluble in ethanol, glycerol, isopropanol, methanol Insoluble in anh. ammonia, diethyl ether |
| Solubility in isopropanol | 14 g/100 g (28 °C) |
| Solubility in methanol | 55 g/100 g (28 °C) |
| Vapor pressure | ~0 mmHg |
| Thermochemistry | |
| Std molar
entropy (S |
79.32 J·mol-1·K-1 |
| Std enthalpy of
formation (ΔfH |
-425.8 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
273 mg/kg (rat, oral) |
| Related compounds | |
| Related alkali hydroxides
|
Lithium hydroxide Sodium hydroxide Rubidium hydroxide Caesium hydroxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potassium hydroxide is a white solid with the formula KOH. It finds many applications in the lab as a general base, though sodium hydroxide is generally preferred as it is easier to find and slightly cheaper.
Contents
Properties
Chemical
Potassium hydroxide dissolves in water and fully dissociates to give a solution of potassium ions and hydroxide ions. Potassium hydroxide solutions will very readily absorb atmospheric carbon dioxide to form potassium carbonate, and must be protected from the atmosphere. Being a strong base, potassium hydroxide also has the ability to dissolve glass (particularly so at higher temperatures) so solution must be kept in a chemically resistant plastic containers, such as high density polyethylene (HDPE).
Potassium hydroxide can be reacted with the corresponding acids to yield potassium salts. Potassium hydroxide is used along with manganese dioxide and an oxidizer to form crude potassium manganate at very high temperatures.
Potassium hydroxide can also be used in a reaction with magnesium powder or shavings in an inert, high boiling solvent with a tertiary alcohol catalyst to yield clean spheres of potassium metal.
Physical
Potassium hydroxide is a waxy white solid which is extremely hygroscopic. It is very soluble in water and its dissolution is highly exothermic. One-hundred percent potassium hydroxide is very difficult to create due to the hygroscopic nature and commercial samples generally contain around 10% water.
Availability
Potassium hydroxide is available reasonably cheaply from soap making suppliers and biodiesel companies such as Duda Diesel. It is also available from Elemental Scientific at a slightly higher cost.
Certain barbecue cleaning products contain 25% KOH, as aqueous solution.
Preparation
Preparation of solid potassium hydroxide is difficult in an amateur setting due to the highly hygroscopic nature of potassium hydroxide.
Historically, potassium hydroxide was made by reacting potassium carbonate (potash) with calcium hydroxide (slaked lime).
- Ca(OH)2 + K2CO3 → CaCO3 + 2 KOH
This method can be performed by the amateur chemist, though it requires corrosion resistant containers when drying or concentrating the potassium hydroxide. Another inconvenient represents the organic residues from the wood ash, which are difficult to remove. They can however be removed completely by roasting the potash in the kiln at very high temperatures in an excess of oxygen.
Heating potassium carbonate at over 891 °C will yield potassium oxide that will react quickly and extremely exothermic with water to form hydroxide. It will also react with any carbon dioxide immediately at lower temperatures. This method is not very practical, as it requires high temperatures.
Electrolysis of a saturated solution of KCl with an ion exchange membrane will also yield potassium hydroxide. This preparation method is similar to that for sodium hydroxide.
Another route, though expensive, involves the reaction of potassium metal or potassium oxide with water.
Projects
- Make potassium permanganate solution
- Make potassium manganate solid
- Produce clean potassium metal
- Make liquid soap
- Make an alkali battery
- Identify certain species of fungi
- Synthesize potassium ferrate
Handling
Safety
Potassium hydroxide is a strong base and is therefore highly caustic, but is not toxic in the sense that it shuts down chemical processes, like arsenic or cyanide. The potassium ion itself is nontoxic when taken orally, but injected into the blood stream it becomes highly toxic and may cause cardiac arrest. The primary danger is saponification of tissue on contact with the hydroxide ions.
Storage
Potassium hydroxide should be stored in closed thick plastic containers, in a dry and cool place, away from any corrosive vapors. Avoid storing it in glass bottles.
Disposal
Potassium hydroxide will turn to potassium carbonate if left in open air, which poses no toxicity to the environment and it's even used as a source of potassium for plants.
Gallery
References
Relevant Sciencemadness threads
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Inorganic compounds
- Potassium compounds
- Bases
- Alkalis
- Hydroxides
- Corrosive chemicals
- Chemicals that attack glassware
- Hygroscopic compounds
- Readily available chemicals
- Essential reagents
- Irritants
- Air-sensitive materials
- Materials unstable in acidic solution
