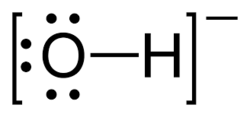Difference between revisions of "Hydroxide"
From Sciencemadness Wiki
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{stub}} | {{stub}} | ||
| − | [[File:OH.png|thumb| | + | [[File:OH drawing.png|thumb|250x250px]] |
| − | Chemical hydroxides are very basic salts. As metal salts, they are usually very insoluble, but | + | Chemical '''hydroxides''' are very basic salts. As metal salts, they are usually very insoluble, but alkali hydroxides are very soluble, inexpensive [[base]]s. |
==Properties== | ==Properties== | ||
| Line 9: | Line 9: | ||
===Physical=== | ===Physical=== | ||
Alkali hydroxides are very soluble and very basic, while other hydroxides are usually only slightly basic and insoluble. | Alkali hydroxides are very soluble and very basic, while other hydroxides are usually only slightly basic and insoluble. | ||
| + | |||
| + | ==Production== | ||
| + | Addition of an alkaline metal oxide to water gives hydroxide. | ||
| + | |||
| + | ==References== | ||
| + | <references/> | ||
| + | ===Relevant Sciencemadness threads=== | ||
[[Category:Anions]] | [[Category:Anions]] | ||
Latest revision as of 18:27, 22 July 2023
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
Chemical hydroxides are very basic salts. As metal salts, they are usually very insoluble, but alkali hydroxides are very soluble, inexpensive bases.
Contents
Properties
Chemical
Hydroxides are usually basic, and can be neutralized by acids (releasing the salt and water).
Physical
Alkali hydroxides are very soluble and very basic, while other hydroxides are usually only slightly basic and insoluble.
Production
Addition of an alkaline metal oxide to water gives hydroxide.