Difference between revisions of "Salicylic acid"
(→Gallery) |
|||
| (6 intermediate revisions by 2 users not shown) | |||
| Line 122: | Line 122: | ||
== Properties == | == Properties == | ||
=== Chemical === | === Chemical === | ||
| − | + | Decarboxylation of salicylic acid via heating will yield [[phenol]]. | |
: C<sub>6</sub>H<sub>4</sub>(OH)COOH → C<sub>6</sub>H<sub>5</sub>OH + CO<sub>2</sub> | : C<sub>6</sub>H<sub>4</sub>(OH)COOH → C<sub>6</sub>H<sub>5</sub>OH + CO<sub>2</sub> | ||
Esterification with [[acetic anhydride]] gives aspirin. | Esterification with [[acetic anhydride]] gives aspirin. | ||
| + | |||
| + | : C<sub>6</sub>H<sub>4</sub>(OH)COOH + (CH<sub>3</sub>O)<sub>2</sub>O → C<sub>6</sub>H<sub>4</sub>(CH<sub>3</sub>OCO)COOH + CH<sub>3</sub>COOH | ||
=== Physical === | === Physical === | ||
| − | Salicylic acid is most often encountered as a fine, fluffy crystalline powder or as needle-like crystals which are difficult to compact. It has a somewhat minty and irritating odor. Salicylic acid is quite soluble in | + | Salicylic acid is most often encountered as a fine, fluffy crystalline powder or as needle-like crystals which are difficult to compact. It has a somewhat minty and irritating odor. Salicylic acid is quite soluble in [[alcohol]]s, [[acetone]], [[diethyl ether]], and slightly lower in nonpolar solvents, such as [[benzene]] and [[toluene]], has low solubility in cold to warm water, and high solubility in boiling water. |
== Availability== | == Availability== | ||
| Line 135: | Line 137: | ||
It can also be found in food stores as preservative, usually as a ester or salt, or sometimes as free acid. Sodium salicylate is more commonly available. The obtain the free acid, simply add a stronger acid to the sodium salt and purify the compound. | It can also be found in food stores as preservative, usually as a ester or salt, or sometimes as free acid. Sodium salicylate is more commonly available. The obtain the free acid, simply add a stronger acid to the sodium salt and purify the compound. | ||
| + | |||
| + | Salicylic acid can be extracted from willow tree bark, by boiling said bark in water, precipitate the impurities, filter, then hydrolyze the resulting salicin to obtain crude salicylic acid. The crude salicylic acid is dissolved in hot water and recrystallized from the solution. Repeated recrystallizations may be needed to remove impurities, namely tannins. This is how salicylic acid was historically prepared. | ||
==Preparation== | ==Preparation== | ||
| Line 141: | Line 145: | ||
Production from aspirin requires the acetylsalicylic acid to be refluxed in the presence of a stronger acid (often [[hydrochloric acid]]) for a period of time, hydrolyzing it to salicylic acid and [[acetic acid]]. The product can then be washed and recrystallized. | Production from aspirin requires the acetylsalicylic acid to be refluxed in the presence of a stronger acid (often [[hydrochloric acid]]) for a period of time, hydrolyzing it to salicylic acid and [[acetic acid]]. The product can then be washed and recrystallized. | ||
| − | |||
| − | |||
Salicylic acid can also be prepared from store-bought oil of wintergreen ([[methyl salicylate]]); a detailed write-up for this process can be found [[Synthesis_of_salicylic_acid#alexleyenda.27s_synthesis_from_methyl_salicylate|here]]. | Salicylic acid can also be prepared from store-bought oil of wintergreen ([[methyl salicylate]]); a detailed write-up for this process can be found [[Synthesis_of_salicylic_acid#alexleyenda.27s_synthesis_from_methyl_salicylate|here]]. | ||
| Line 148: | Line 150: | ||
== Projects == | == Projects == | ||
* Make aspirin | * Make aspirin | ||
| + | * Make Pepto-Bismol (bismuth subsalicylate) | ||
* [[Esterification]] of [[salicylic acid]] with [[methanol]] to make [[methyl salicylate]] | * [[Esterification]] of [[salicylic acid]] with [[methanol]] to make [[methyl salicylate]] | ||
| − | * Reaction of salicylic acid with carbamide and boric acid to form Salicylamide | + | * Reaction of salicylic acid with [[urea|carbamide]] and [[boric acid]] to form Salicylamide |
* Make [[phenol]] | * Make [[phenol]] | ||
* Make Salsalate | * Make Salsalate | ||
| + | * Skin exfoliation | ||
==Handling== | ==Handling== | ||
| Line 158: | Line 162: | ||
===Storage=== | ===Storage=== | ||
| − | No special storage is required, salicylic acid can be stored in any clean container. | + | No special storage is required, salicylic acid can be stored in any clean container, plastic or glass. |
===Disposal=== | ===Disposal=== | ||
Salicylic acid can be safely poured down the drain or dumped in the trash, as it does not pose any danger for the environment. | Salicylic acid can be safely poured down the drain or dumped in the trash, as it does not pose any danger for the environment. | ||
| − | == Gallery == | + | ==Gallery== |
| − | + | <gallery widths="200" position="center" columns="4" orientation="none"> | |
| + | Salicylic acid recrystallized by Alexleyenda.jpg|Fine, needle-like crystals of salicylic acid produced through recrystallization. | ||
| + | Sali1.jpg|A reflux setup for producing salicylic acid by [[Synthesis_of_salicylic_acid#alexleyenda.27s_synthesis_from_methyl_salicylate|Alexleyenda's process]]. | ||
| + | Salicylic_acid_crystals.jpg|Needle shaped crystals of salicylic acid | ||
| + | </gallery> | ||
==References== | ==References== | ||
| Line 171: | Line 179: | ||
*[http://www.sciencemadness.org/talk/viewthread.php?tid=10914 Salicylic acid] | *[http://www.sciencemadness.org/talk/viewthread.php?tid=10914 Salicylic acid] | ||
*[http://sciencemadness.org/talk/viewthread.php?tid=11753 preparation of salicylic acid from anthranilic acid] | *[http://sciencemadness.org/talk/viewthread.php?tid=11753 preparation of salicylic acid from anthranilic acid] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=31818 Salicylic Acid Hydrolysis] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=1413 salicylic acid] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=6363 Obtaining Acetic acid/Salicylic acid from aspirin] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=68210 Hollow salycylic acid crystals !?] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=23300 Salicylic acid solution turning pink] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=63935 Why does wet Salicylic Acid turn black?] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=64887 DMSO + Salicylic Acid? (Wart Removal)] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=11314 oxidation of salicylic acid] | ||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
Latest revision as of 12:29, 25 June 2023
 Hair-like crystals of salicylic acid in the bottom of a beaker. (Click to see up close)
| |
| |
| Names | |
|---|---|
| IUPAC name
2-Hydroxybenzoic acid
| |
| Other names
2-Carboxyphenol
O-Carboxyphenol O-hydroxybenzoic acid | |
| Properties | |
| C7H6O3 | |
| Molar mass | 138.12 g/mol |
| Appearance | Colorless crystalline solid |
| Odor | Odorless |
| Density | 1.443 g/cm3 (20 °C) |
| Melting point | 158.6 °C (317.5 °F; 431.8 K) |
| Boiling point | 200 °C (392 °F; 473 K) (decomposes) |
| 0.124 g/100 ml (0 °C) 0.248 g/100 ml (25 °C) 0.414 g/100 ml (40 °C) 1.741 g/100 ml (75 °C) 7.779 g/100 ml (100 °C) | |
| Solubility | Soluble in acetone, chloroform, diethyl ether, ethanol, methanol, propanol, oil of turpentine Slightly soluble in benzene, CCl4, toluene |
| Solubility in acetone | 39.6 g/100 g (23 °C) |
| Solubility in benzene | 0.46 g/100 g (11.7 °C) 0.775 g/100 g (25 °C) 0.991 g/100 g (30.5 °C) 2.38 g/100 g (49.4 °C) 4.4 g/100 g (64.2 °C) |
| Solubility in chloroform | 2.22 g/100 ml (25 °C) 2.31 g/100 ml (30.5 °C) |
| Solubility in methanol | 40.67 g/100 g (−3 °C) 62.48 g/100 g (21 °C) |
| Solubility in olive oil | 2.43 g/100 g (23 °C) |
| Vapor pressure | 8.2·10-5 mmHg (25 °C) |
| Acidity (pKa) | 1 = 2.97 (25 °C) 2 = 13.82 (20 °C) |
| Thermochemistry | |
| Std enthalpy of
formation (ΔfH |
-589.9 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 157 °C (315 °F; 430 K) (closed cup) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
480 mg/kg (mice, oral) |
| Related compounds | |
| Related compounds
|
Acetylsalicylic acid Phenol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
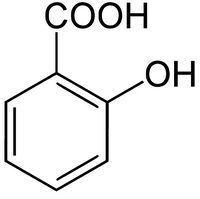
Salicylic acid is the organic compound with the chemical formula C6H4(OH)COOH. It is an example of a phenolic acid. It is the active metabolite of aspirin and is also used in many other medications such as skin-care products.
Contents
Properties
Chemical
Decarboxylation of salicylic acid via heating will yield phenol.
- C6H4(OH)COOH → C6H5OH + CO2
Esterification with acetic anhydride gives aspirin.
- C6H4(OH)COOH + (CH3O)2O → C6H4(CH3OCO)COOH + CH3COOH
Physical
Salicylic acid is most often encountered as a fine, fluffy crystalline powder or as needle-like crystals which are difficult to compact. It has a somewhat minty and irritating odor. Salicylic acid is quite soluble in alcohols, acetone, diethyl ether, and slightly lower in nonpolar solvents, such as benzene and toluene, has low solubility in cold to warm water, and high solubility in boiling water.
Availability
Salicylic acid, while usually produced using aspirin as a precursor with ease, can also be purchased in solution as various skin-care products for treating warts or acne, those these can be both expensive and impure, as well as highly diluted.
It can also be found in food stores as preservative, usually as a ester or salt, or sometimes as free acid. Sodium salicylate is more commonly available. The obtain the free acid, simply add a stronger acid to the sodium salt and purify the compound.
Salicylic acid can be extracted from willow tree bark, by boiling said bark in water, precipitate the impurities, filter, then hydrolyze the resulting salicin to obtain crude salicylic acid. The crude salicylic acid is dissolved in hot water and recrystallized from the solution. Repeated recrystallizations may be needed to remove impurities, namely tannins. This is how salicylic acid was historically prepared.
Preparation
Salicylic acid can be easily synthesized using either methyl salicylate or acetylsalicylic acid (aspirin) as the primary precursor, but because methyl salicylate may not be as cheaply or easily obtained as aspirin, it is usually synthesized from the latter, which is also typically seen as the easier process.
Production from aspirin requires the acetylsalicylic acid to be refluxed in the presence of a stronger acid (often hydrochloric acid) for a period of time, hydrolyzing it to salicylic acid and acetic acid. The product can then be washed and recrystallized.
Salicylic acid can also be prepared from store-bought oil of wintergreen (methyl salicylate); a detailed write-up for this process can be found here.
Projects
- Make aspirin
- Make Pepto-Bismol (bismuth subsalicylate)
- Esterification of salicylic acid with methanol to make methyl salicylate
- Reaction of salicylic acid with carbamide and boric acid to form Salicylamide
- Make phenol
- Make Salsalate
- Skin exfoliation
Handling
Safety
Salicylic acid has the ability to break down lipids in the skin, causing symptoms ranging from dryness and irritation at low concentrations to mild acid burns at higher ones. When ingested orally in large amounts it can cause salicylate intoxication, which may produce very serious side effects.
Storage
No special storage is required, salicylic acid can be stored in any clean container, plastic or glass.
Disposal
Salicylic acid can be safely poured down the drain or dumped in the trash, as it does not pose any danger for the environment.
Gallery
A reflux setup for producing salicylic acid by Alexleyenda's process.
References
Relevant Sciencemandess threads
- Salicylic acid
- preparation of salicylic acid from anthranilic acid
- Salicylic Acid Hydrolysis
- salicylic acid
- Obtaining Acetic acid/Salicylic acid from aspirin
- Hollow salycylic acid crystals !?
- Salicylic acid solution turning pink
- Why does wet Salicylic Acid turn black?
- DMSO + Salicylic Acid? (Wart Removal)
- oxidation of salicylic acid


