Difference between revisions of "Nitromethane"
(→Preparation) |
|||
| (11 intermediate revisions by 3 users not shown) | |||
| Line 8: | Line 8: | ||
| OtherNames = Nitro fuel<br>Nitrocarbol | | OtherNames = Nitro fuel<br>Nitrocarbol | ||
<!-- Images --> | <!-- Images --> | ||
| − | | ImageFile = | + | | ImageFile = Nitromethane.jpg |
| − | | ImageSize = | + | | ImageSize = 270px |
| ImageAlt = | | ImageAlt = | ||
| ImageName = | | ImageName = | ||
| − | | ImageFile1 = | + | | ImageFile1 = Nitromethane structural formula.png |
| − | | ImageSize1 = | + | | ImageSize1 = 200px |
| ImageAlt1 = | | ImageAlt1 = | ||
| ImageName1 = | | ImageName1 = | ||
| Line 44: | Line 44: | ||
| 3DMet = | | 3DMet = | ||
| Abbreviations = | | Abbreviations = | ||
| − | | SMILES = | + | | SMILES = C[N+](=O)[O-] |
}} | }} | ||
| Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| Line 65: | Line 65: | ||
| pKa = 17.2 ([[Dimethyl sulfoxide|DMSO]]) | | pKa = 17.2 ([[Dimethyl sulfoxide|DMSO]]) | ||
| pKb = | | pKb = | ||
| − | | Solubility = | + | | Solubility = 9.5 ml/100 ml (1.8%) (20 °C)<br>2.1% (20 °C)<br>7.6% (70 °C) |
| − | | SolubleOther = Reacts with amines and other bases<br>Miscible with [[acetone]], [[carbon tetrachloride]], [[diethyl ether]], [[ethanol]], [[methanol]] | + | | SolubleOther = Reacts with amines and other bases<br>Miscible with [[acetone]], [[carbon tetrachloride]], [[diethyl ether]], [[ethanol]], [[methanol]]<br>Immiscible with alkanes, [[ethylene glycol]], [[glycerol]] |
| Solvent = | | Solvent = | ||
| VaporPressure = 28 mmHg (20 °C) | | VaporPressure = 28 mmHg (20 °C) | ||
| Line 109: | Line 109: | ||
}} | }} | ||
}} | }} | ||
| − | '''Nitromethane''' is an organic compound with the chemical formula '''CH<sub>3</sub>NO<sub>2</sub>'''. It is the simplest organic nitro compound. | + | '''Nitromethane''' ('''NM''') is an organic compound with the chemical formula '''CH<sub>3</sub>NO<sub>2</sub>'''. It is the simplest organic nitro compound. |
==Properties== | ==Properties== | ||
| Line 121: | Line 121: | ||
: NaOH + CH<sub>3</sub>NO<sub>2</sub> → NaCH<sub>2</sub>=NO<sub>2</sub> + H<sub>2</sub>O | : NaOH + CH<sub>3</sub>NO<sub>2</sub> → NaCH<sub>2</sub>=NO<sub>2</sub> + H<sub>2</sub>O | ||
| − | The heat generated by the reaction can even ignite the nitromethane.<ref>https://www.youtube.com/watch?v=r3CjogY5T0I</ref> | + | The heat generated by the reaction can even ignite the nitromethane.<ref>'''Eli Tyo''' (2009), ''Two liquids reacting violently.'', https://www.youtube.com/watch?v=r3CjogY5T0I</ref> Solid sodium nitronate can be obtained by performing the reaction in ethanolic sodium hydroxide or ethoxide.<ref>'''ExplosionsAndFire''' (2019), ''Nitromethane'', https://www.bitchute.com/video/vhhXK6PeDIc2/</ref> |
| − | Nitromethane will not react with strong oxidizers like [[manganese heptoxide]]. This was observed by many SM members, such as Rhodanide and Tdep. | + | Nitromethane will not react with strong oxidizers like [[manganese heptoxide]]. This was observed by many SM members, such as Rhodanide and Tdep. |
| + | |||
| + | When refluxed with mineral acids, nitromethane, much like higher primary nitroparaffins, decomposes to form hydroxylamine salts:<ref>S. B. Lippincott, H. B. Hass, ''Ind. Eng. Chem.'' '''1939''', 31, 1, 118–120, https://doi.org/10.1021/ie50349a025</ref><ref>N. Kornblum, R. A. Brown, ''J. Am. Chem. Soc.'' '''1965''', 87, 8, 1742–1747, https://doi.org/10.1021/ja01086a023</ref> | ||
| + | |||
| + | : CH<sub>3</sub>NO<sub>2</sub> + HCl + H<sub>2</sub>O → [NH<sub>3</sub>OH]Cl + HCOOH | ||
===Physical=== | ===Physical=== | ||
Nitromethane is a colorless, slightly viscous, highly polar liquid. It is poorly soluble in water, but miscible with other organic solvents, such as alcohols. It freezes at −29 °C and boils at around 100 °C. | Nitromethane is a colorless, slightly viscous, highly polar liquid. It is poorly soluble in water, but miscible with other organic solvents, such as alcohols. It freezes at −29 °C and boils at around 100 °C. | ||
| + | |||
| + | Nitromethane will form a heteroazeotrope with water, consisting of 76.4% NM by weight, which boils at 83.6°C. It forms also azeotropes with other solvents, most importantly methanol (8% NM at 64.5 °C) and ethanol (26.8% NM at 76 °C).<ref>Azeotropes beginning with N, http://homepages.ed.ac.uk/jwp/Chemeng/azeotrope/NN.html</ref> Other sources list the methanol azeotrope as 9% NM boiling at 64.6 °C,<ref>Azeotrope tables, https://en.wikipedia.org/wiki/Azeotrope_tables</ref> 9% NM boiling at 64.4 °C<ref>K. Nakanishi, H. Shirai, K. Nakasato, ''J. Chem. Eng. Data'' '''1968''', 13, 2, 188–191, https://doi.org/10.1021/je60037a013</ref> and as 10.5% NM boiling at 64.55 °C.<ref>G. Desseigne, C. Belliot, ''J. Chim. Phys.'' '''1952''', 49, 46–48, https://doi.org/10.1051/jcp/1952490046</ref> | ||
==Availability== | ==Availability== | ||
| − | Nitromethane is often available from lab suppliers at a price of around $100/L. | + | Nitromethane is often available from lab suppliers at a price of around $100/L. In the EU, the sell of pure NM is restricted due to its potential use in terrorist activities, and the maximum concentration legally available OTC is 30%. |
| − | To separate nitromethane from RC fuel, you will first have to fractionally distill the RC fuel. The first fraction that will distill is the NM-MeOH azeotrope, containing 9% NM at 64.6 °C, which is close to the boiling point of pure methanol at 64.7 °C, meaning that unless you have a very long column or a very efficient fractionating system, you will practically distill the MeOH along with the NM-MeOH azeotrope. However, if your RC fuel has high NM percentage, the leftover NM will distill next, leaving the castor oil and other high boiling point fractions in the flask. Separation of NM from the NM-MeOH azeotrope can be done by adding a neutral/acidic salt or acid to the mixture, which should salt out the NM from the MeOH. The separated NM is purified via distillation. The NM obtained this way will still have some methanol present, and removing the last bits of methanol is not necessary for most purposes, as they do | + | It is also sold in many places locally for use in RC fuel, either pure or as a mixture with [[methanol]] and (castor) oil. Separation of the nitromethane from this mixture can be quite difficult, but it is achievable with good technique. |
| + | |||
| + | To separate nitromethane from RC fuel, you will first have to fractionally distill the RC fuel. The first fraction that will distill is the NM-MeOH azeotrope, containing 9% NM at 64.6 °C, which is close to the boiling point of pure methanol at 64.7 °C, meaning that unless you have a very long column or a very efficient fractionating system, you will practically distill the MeOH along with the NM-MeOH azeotrope. However, if your RC fuel has a high NM percentage, the leftover NM will distill next, leaving the castor oil and other high boiling point fractions in the flask (vacuum distillation can be benefitial at this point as it prevents thermal breakdown of the oil). Separation of NM from the NM-MeOH azeotrope can be done by adding a neutral/acidic salt or acid to the mixture, which should salt out the NM from the MeOH, though this doesn't always work.<sup>''[citation needed]''</sup> It has been claimed that the nitromethane can be easily extracted from the methanol azeotrope by freezing it out with dry ice and filtering it over a frit packed with dry ice pellets, in analogy to how it can be recovered from ethereal solutions.<ref>F. William Parrett, M. Shin Sun, ''J. Chem. Educ.'' '''1977''', 54, 7, 448, https://doi.org/10.1021/ed054p448</ref> The separated NM is purified via distillation. The NM obtained this way will still have some methanol present, and removing the last bits of methanol is not necessary for most purposes, as they do not interfere with reactions. | ||
==Preparation== | ==Preparation== | ||
Nitromethane is produced industrially by treating [[propane]] with [[nitric acid]] at 350–450 °C. This exothermic reaction produces the four industrially significant nitroalkanes: nitromethane, [[nitroethane]], 1-nitropropane, and 2-nitropropane. Nitromethane is then separated via fractional distillation. | Nitromethane is produced industrially by treating [[propane]] with [[nitric acid]] at 350–450 °C. This exothermic reaction produces the four industrially significant nitroalkanes: nitromethane, [[nitroethane]], 1-nitropropane, and 2-nitropropane. Nitromethane is then separated via fractional distillation. | ||
| − | + | A more accessible method involves the reaction between sodium chloroacetate, obtained by carefully neutralizing [[chloroacetic acid]] with sodium hydroxide, carbonate or bicarbonate and [[sodium nitrite]] in aqueous solution. This forms sodium nitroacetate, which decomposes when heated to 80-85°, its decomposition at this temperature produces enough heat that it no longer needs external heat:<ref>http://www.orgsyn.org/demo.aspx?prep=CV1P0401</ref> | |
| − | :ClCH<sub>2</sub>COONa + NaNO<sub>2</sub> + H<sub>2</sub>O → CH<sub>3</sub>NO<sub>2</sub> + NaCl + NaHCO<sub>3</sub> | + | |
| + | : ClCH<sub>2</sub>COONa + NaNO<sub>2</sub> + H<sub>2</sub>O → CH<sub>3</sub>NO<sub>2</sub> + NaCl + NaHCO<sub>3</sub> | ||
==Projects== | ==Projects== | ||
| Line 178: | Line 187: | ||
[[Category:Energetic materials]] | [[Category:Energetic materials]] | ||
[[Category:Materials unstable in basic solution]] | [[Category:Materials unstable in basic solution]] | ||
| + | [[Category:Liquids]] | ||
Latest revision as of 20:01, 16 May 2023
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|

| |
| |
| Names | |
|---|---|
| IUPAC name
Nitromethane
| |
| Other names
Nitro fuel
Nitrocarbol | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| CH3NO2 | |
| Molar mass | 61.04 g/mol |
| Appearance | Colorless liquid |
| Odor | Fruity, pungent |
| Density | 1.1371 g/cm3 (20 °C) |
| Melting point | −28.38 °C (−19.08 °F; 244.77 K) |
| Boiling point | 101.19 °C (214.14 °F; 374.34 K) |
| 9.5 ml/100 ml (1.8%) (20 °C) 2.1% (20 °C) 7.6% (70 °C) | |
| Solubility | Reacts with amines and other bases Miscible with acetone, carbon tetrachloride, diethyl ether, ethanol, methanol Immiscible with alkanes, ethylene glycol, glycerol |
| Vapor pressure | 28 mmHg (20 °C) |
| Acidity (pKa) | 17.2 (DMSO) |
| Thermochemistry | |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 35 °C (95 °F; 308 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
940 mg/kg (rat, oral) 950 mg/kg (mouse, oral) |
| Related compounds | |
| Related compounds
|
Nitroethane |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
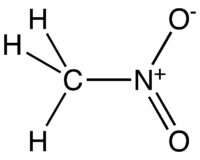
Nitromethane (NM) is an organic compound with the chemical formula CH3NO2. It is the simplest organic nitro compound.
Contents
Properties
Chemical
Nitromethane burns when ignited in air, releasing combustion gasses. It burns with a gray flame.
- 4 CH3NO2 + 3 O2 → 4 CO2 + 6 H2O + 2 N2
Nitromethane reacts violently with bases, such as sodium hydroxide or ammonia/amines, forming nitronate salts, which are highly sensitive to shock and impact, moreso when dry.
- NaOH + CH3NO2 → NaCH2=NO2 + H2O
The heat generated by the reaction can even ignite the nitromethane.[1] Solid sodium nitronate can be obtained by performing the reaction in ethanolic sodium hydroxide or ethoxide.[2]
Nitromethane will not react with strong oxidizers like manganese heptoxide. This was observed by many SM members, such as Rhodanide and Tdep.
When refluxed with mineral acids, nitromethane, much like higher primary nitroparaffins, decomposes to form hydroxylamine salts:[3][4]
- CH3NO2 + HCl + H2O → [NH3OH]Cl + HCOOH
Physical
Nitromethane is a colorless, slightly viscous, highly polar liquid. It is poorly soluble in water, but miscible with other organic solvents, such as alcohols. It freezes at −29 °C and boils at around 100 °C.
Nitromethane will form a heteroazeotrope with water, consisting of 76.4% NM by weight, which boils at 83.6°C. It forms also azeotropes with other solvents, most importantly methanol (8% NM at 64.5 °C) and ethanol (26.8% NM at 76 °C).[5] Other sources list the methanol azeotrope as 9% NM boiling at 64.6 °C,[6] 9% NM boiling at 64.4 °C[7] and as 10.5% NM boiling at 64.55 °C.[8]
Availability
Nitromethane is often available from lab suppliers at a price of around $100/L. In the EU, the sell of pure NM is restricted due to its potential use in terrorist activities, and the maximum concentration legally available OTC is 30%.
It is also sold in many places locally for use in RC fuel, either pure or as a mixture with methanol and (castor) oil. Separation of the nitromethane from this mixture can be quite difficult, but it is achievable with good technique.
To separate nitromethane from RC fuel, you will first have to fractionally distill the RC fuel. The first fraction that will distill is the NM-MeOH azeotrope, containing 9% NM at 64.6 °C, which is close to the boiling point of pure methanol at 64.7 °C, meaning that unless you have a very long column or a very efficient fractionating system, you will practically distill the MeOH along with the NM-MeOH azeotrope. However, if your RC fuel has a high NM percentage, the leftover NM will distill next, leaving the castor oil and other high boiling point fractions in the flask (vacuum distillation can be benefitial at this point as it prevents thermal breakdown of the oil). Separation of NM from the NM-MeOH azeotrope can be done by adding a neutral/acidic salt or acid to the mixture, which should salt out the NM from the MeOH, though this doesn't always work.[citation needed] It has been claimed that the nitromethane can be easily extracted from the methanol azeotrope by freezing it out with dry ice and filtering it over a frit packed with dry ice pellets, in analogy to how it can be recovered from ethereal solutions.[9] The separated NM is purified via distillation. The NM obtained this way will still have some methanol present, and removing the last bits of methanol is not necessary for most purposes, as they do not interfere with reactions.
Preparation
Nitromethane is produced industrially by treating propane with nitric acid at 350–450 °C. This exothermic reaction produces the four industrially significant nitroalkanes: nitromethane, nitroethane, 1-nitropropane, and 2-nitropropane. Nitromethane is then separated via fractional distillation.
A more accessible method involves the reaction between sodium chloroacetate, obtained by carefully neutralizing chloroacetic acid with sodium hydroxide, carbonate or bicarbonate and sodium nitrite in aqueous solution. This forms sodium nitroacetate, which decomposes when heated to 80-85°, its decomposition at this temperature produces enough heat that it no longer needs external heat:[10]
- ClCH2COONa + NaNO2 + H2O → CH3NO2 + NaCl + NaHCO3
Projects
- Methyl nitroacetate synthesis
- RC fuel
- Make ANNM
- Facile synthesis of hydroxylammonium salts
- Dissolve superglue
Handling
Safety
Nitromethane is quite inflammable so it should not be handled around open flames or other ignition sources. Pure nitromethane is a powerful explosive, though it is rather insensitive and difficult to initiate. Gloves should be worn when working with nitromethane to prevent accidental skin contact.
Storage
Nitromethane must be kept in closed bottles away from any ignition sources and away from ammonia and other amines.
Disposal
Nitromethane can be safely burned, though it's best to mix it with another fuel first, like ethanol, as the flame is nearly invisible.
For the inexperienced chemist, the text below shows the dangers of handling nitromethane without knowing its properties:
Aqueous solution of sodium hydroxide will readily hydrolyze it to methanol, which can be safely burned.
- No. The chemistry of C-Nitro compounds is very different to nitrite and nitrate esters. Hydrolysis of nitromethane by sodium hydroxide is messy and exothermic through potentially explosive thermal and shock sensitive salts/compounds ultimately to carbonate and ammonia. Bases are sensitisers of nitromethane. This is not a good plan. Marvin (talk) 12:42, 9 July 2016 (UTC)
References
- ↑ Eli Tyo (2009), Two liquids reacting violently., https://www.youtube.com/watch?v=r3CjogY5T0I
- ↑ ExplosionsAndFire (2019), Nitromethane, https://www.bitchute.com/video/vhhXK6PeDIc2/
- ↑ S. B. Lippincott, H. B. Hass, Ind. Eng. Chem. 1939, 31, 1, 118–120, https://doi.org/10.1021/ie50349a025
- ↑ N. Kornblum, R. A. Brown, J. Am. Chem. Soc. 1965, 87, 8, 1742–1747, https://doi.org/10.1021/ja01086a023
- ↑ Azeotropes beginning with N, http://homepages.ed.ac.uk/jwp/Chemeng/azeotrope/NN.html
- ↑ Azeotrope tables, https://en.wikipedia.org/wiki/Azeotrope_tables
- ↑ K. Nakanishi, H. Shirai, K. Nakasato, J. Chem. Eng. Data 1968, 13, 2, 188–191, https://doi.org/10.1021/je60037a013
- ↑ G. Desseigne, C. Belliot, J. Chim. Phys. 1952, 49, 46–48, https://doi.org/10.1051/jcp/1952490046
- ↑ F. William Parrett, M. Shin Sun, J. Chem. Educ. 1977, 54, 7, 448, https://doi.org/10.1021/ed054p448
- ↑ http://www.orgsyn.org/demo.aspx?prep=CV1P0401
Relevant Sciencemadness threads
- Article stubs
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Nitro compounds
- Nitroalkanes
- Solvents
- Polar solvents
- Protic solvents
- Energetic materials
- Materials unstable in basic solution
- Liquids