Difference between revisions of "Allyl alcohol"
| (2 intermediate revisions by the same user not shown) | |||
| Line 5: | Line 5: | ||
| PIN = | | PIN = | ||
| SystematicName = | | SystematicName = | ||
| − | | OtherNames = 1-Propen-3-ol<br> | + | | OtherNames = 1-Propen-3-ol<br>Allylalcohol<br>Vinyl carbinol |
<!-- Images --> | <!-- Images --> | ||
| ImageFile = | | ImageFile = | ||
| Line 11: | Line 11: | ||
| ImageAlt = | | ImageAlt = | ||
| ImageName = | | ImageName = | ||
| − | | ImageFile1 = | + | | ImageFile1 = Allylalcohol structure.png |
| − | | ImageSize1 = | + | | ImageSize1 = 180 |
| ImageAlt1 = | | ImageAlt1 = | ||
| ImageName1 = | | ImageName1 = | ||
| Line 76: | Line 76: | ||
| Section4 = {{Chembox Thermochemistry | | Section4 = {{Chembox Thermochemistry | ||
| DeltaGf = | | DeltaGf = | ||
| − | | DeltaHc = | + | | DeltaHc = 1,853.8 kJ/mol |
| DeltaHf = | | DeltaHf = | ||
| Entropy = | | Entropy = | ||
| Line 113: | Line 113: | ||
===Chemical=== | ===Chemical=== | ||
Epoxidation of allyl alcohol yields glycidol. | Epoxidation of allyl alcohol yields glycidol. | ||
| + | |||
| + | Allyl alcohol will isomerize into ketone by the action of the Grubbs reagent. | ||
| + | |||
| + | Strong oxidizers, like peroxides may cause polymerization of allyl alcohol. | ||
===Physical=== | ===Physical=== | ||
| Line 131: | Line 135: | ||
==Projects== | ==Projects== | ||
*Make glycidol | *Make glycidol | ||
| + | *Make allyl halide | ||
==Handling== | ==Handling== | ||
Latest revision as of 20:21, 15 September 2022
| |
| Names | |
|---|---|
| IUPAC name
Prop-2-en-1-ol
| |
| Other names
1-Propen-3-ol
Allylalcohol Vinyl carbinol | |
| Properties | |
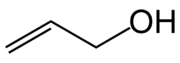
| C3H6O CH2=CHCH2OH | |
| Molar mass | 58.08 g/mol |
| Appearance | Colorless liquid |
| Odor | Mustard-like |
| Density | 0.854 g/cm3 (25 °C) |
| Melting point | −129 °C (−200 °F; 144 K) |
| Boiling point | 97 °C (207 °F; 370 K) |
| Misicible | |
| Solubility | Misicible with acetone, benzene, chloroform, diethyl ether, dichloromethane, isopropanol, DMSO, petroleum ether, toluene |
| Vapor pressure | 23.8 mmHg (25 °C) |
| Acidity (pKa) | 15.5 (H2O) |
| Thermochemistry | |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 21 °C (70 °F; 294 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
64 mg/kg (rat, oral) |
| Related compounds | |
| Related compounds
|
Propanol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Allyl alcohol or prop-2-en-1-ol is an organic compound with the structural formula CH2=CHCH2OH. Allyl alcohol is the smallest representative of the allylic alcohols
Contents
Properties
Chemical
Epoxidation of allyl alcohol yields glycidol.
Allyl alcohol will isomerize into ketone by the action of the Grubbs reagent.
Strong oxidizers, like peroxides may cause polymerization of allyl alcohol.
Physical
Allyl alcohol is a colorless liquid, with a mustard-like odor. It is miscible with water and organic solvents.
Availability
Allyl alcohol is sold by lab suppliers, but it's difficult to acquire as it's quite toxic.
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.
Preparation
Allyl alcohol can be obtained by heating glycerol with oxalic acid at high temperatures (around 220-240 °C). Ammonium chloride may also be added as catalyst. The first product of this reaction is formic acid (this is a convenient route to obtain formic acid). After no more formic acid is produced, at temperatures between 220-225 °C, an equal amount of allyl alcohol and allyl formate distills over. The distillate was treated with sodium or potassium hydroxide to hydrolyze the formate and destroy any traces of acrolein produced, allowed to stand for 12h at room temp and finally distilled. The first distillate contains all the allyl alcohol, in the form of allyl alcohol/water azeotropic mixture (b.p. 87-88 °C) which may be dehydrated using anhydrous potassium carbonate to obtain anhydrous allyl alcohol.[1]
Alternatively, glycerol can be distilled with formic acid alone, and the purification process is similar to that above.[2][3]
Allyl alcohol can also be made by the rearrangement of propylene oxide, a reaction that is catalyzed by potassium alum at high temperature.
Projects
- Make glycidol
- Make allyl halide
Handling
Safety
Allyl alcohol is more toxic than related simple alcohols. Its threshold limit value (TLV) is 2 ppm. It is also a lachrymator.
When handling this compound, it's advised to use butyl rubber gloves, as they provide the best protection.
Storage
Allyl alcohol should be kept in airtight bottles, with a clear hazard label, in dark well ventilated places.
Disposal
Allyl alcohol should be diluted with a flammable solvent, like ethanol or acetone and burned in an incinerator, kiln, outside or in a special fumehood.
References
- ↑ https://www.erowid.org/archive/rhodium/chemistry/allylalcohol.html
- ↑ http://www.orgsyn.org/demo.aspx?prep=cv1p0042
- ↑ https://www.prepchem.com/synthesis-of-allyl-alcohol/