Difference between revisions of "Calcium gluconate"
(→Availability and Uses) |
|||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | '''Calcium gluconate''' is a calcium salt of gluconic acid. Among chemists, it is best known as an antidote for [[hydrofluoric acid]] exposure. | + | {{Chembox |
| + | | Name = Calcium gluconate | ||
| + | | Reference = | ||
| + | | IUPACName = Calcium (2R,3S,4R,5R)- 2,3,4,5,6-pentahydroxyhexanoate | ||
| + | | PIN = | ||
| + | | SystematicName = | ||
| + | | OtherNames = Calciofon<br>Calcium D-gluconate<br>Calglucon<br>E578<br>Glucobiogen | ||
| + | <!-- Images --> | ||
| + | | ImageFile = | ||
| + | | ImageSize = | ||
| + | | ImageAlt = | ||
| + | | ImageName = | ||
| + | | ImageFile1 = Calcium gluconate structure.png | ||
| + | | ImageSize1 = 180 | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageFile2 = | ||
| + | | ImageSize2 = | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = | ||
| + | | ImageSizeL1 = | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| + | | Section1 = {{Chembox Identifiers | ||
| + | | 3DMet = | ||
| + | | Abbreviations = | ||
| + | | SMILES = | ||
| + | }} | ||
| + | | Section2 = {{Chembox Properties | ||
| + | | AtmosphericOHRateConstant = | ||
| + | | Appearance = White solid | ||
| + | | BoilingPt = | ||
| + | | BoilingPtC = | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = Decomposes | ||
| + | | Density = | ||
| + | | Formula = C<sub>12</sub>H<sub>22</sub>O<sub>14</sub>Ca | ||
| + | | HenryConstant = | ||
| + | | LogP = | ||
| + | | MolarMass = 430.372 g/mol | ||
| + | | MeltingPt = | ||
| + | | MeltingPtC = 178 | ||
| + | | MeltingPt_ref = | ||
| + | | MeltingPt_notes = (decomposes)<ref>Nilkantum; J.Sci.Technol.India; vol. 2; (1936); p. 39; Chem.Abstr.; (1938); p. 1403</ref> | ||
| + | | Odor = Odorless | ||
| + | | pKa = | ||
| + | | pKb = | ||
| + | | Solubility = 3.5 g/100 ml (25 °C) | ||
| + | | SolubleOther = Insoluble in glacial [[acetic acid]], [[diethyl ether]] | ||
| + | | Solvent = | ||
| + | | VaporPressure = ~0 mmHg | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = | ||
| + | | CrystalStruct = | ||
| + | | MolShape = | ||
| + | }} | ||
| + | | Section4 = {{Chembox Thermochemistry | ||
| + | | DeltaGf = | ||
| + | | DeltaHc = | ||
| + | | DeltaHf = | ||
| + | | Entropy = | ||
| + | | HeatCapacity = | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = | ||
| + | | FrictionSens = | ||
| + | | DetonationV = | ||
| + | | REFactor = | ||
| + | }} | ||
| + | | Section6 = {{Chembox Hazards | ||
| + | | AutoignitionPt = | ||
| + | | ExploLimits = | ||
| + | | ExternalMSDS = [https://www.docdroid.net/xCM0TIm/calcium-gluconate-sa.pdf.html Sigma-Aldrich] (monohydrate) | ||
| + | | FlashPt = | ||
| + | | LD50 = | ||
| + | | LC50 = | ||
| + | | MainHazards = Irritant | ||
| + | | NFPA-F = | ||
| + | | NFPA-H = | ||
| + | | NFPA-R = | ||
| + | | NFPA-S = | ||
| + | }} | ||
| + | | Section7 = {{Chembox Related | ||
| + | | OtherAnions = | ||
| + | | OtherCations = | ||
| + | | OtherFunction = | ||
| + | | OtherFunction_label = | ||
| + | | OtherCompounds = | ||
| + | }} | ||
| + | }} | ||
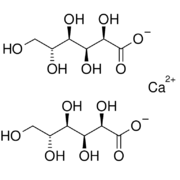
| + | '''Calcium gluconate''' is a calcium salt of [[gluconic acid]]. Among chemists, it is best known as an [[antidote]] for [[hydrofluoric acid]] exposure. | ||
== Properties == | == Properties == | ||
=== Physical === | === Physical === | ||
| − | White powder, moderately soluble in cold water, readily soluble in hot water. | + | White powder, moderately soluble in cold [[water]], readily soluble in hot water. It melts with decomposition around 178 °C, though some sources claim 120 °C. |
=== Chemical === | === Chemical === | ||
| Line 12: | Line 121: | ||
Its main medical use is as a calcium supplement. Chemists use it as an antidote for [[hydrofluoric acid]] poisoning, in forms of topical creamy gel or injections. | Its main medical use is as a calcium supplement. Chemists use it as an antidote for [[hydrofluoric acid]] poisoning, in forms of topical creamy gel or injections. | ||
| + | |||
| + | A 25% calcium gluconate aq. solution can be purchased from some farm and veterinary supplies, where it is intended for the treatment of milk fever. <ref>http://www.pbsanimalhealth.com/details/Calcium-Gluconate-23%25-Solution/13-86.html</ref> | ||
== Projects == | == Projects == | ||
| Line 25: | Line 136: | ||
=== Disposal === | === Disposal === | ||
| − | Calcium gluconate can be disposed of into the ground or drain safely. | + | Calcium gluconate can be disposed of into the ground, trash or drain safely. |
| + | |||
| + | ==References== | ||
| + | <references/> | ||
| + | ===Relevant Sciencemadness threads=== | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=3176 Calcium gluconate preparation......] | ||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
Latest revision as of 20:10, 15 September 2022
| |
| Names | |
|---|---|
| IUPAC name
Calcium (2R,3S,4R,5R)- 2,3,4,5,6-pentahydroxyhexanoate
| |
| Other names
Calciofon
Calcium D-gluconate Calglucon E578 Glucobiogen | |
| Properties | |
| C12H22O14Ca | |
| Molar mass | 430.372 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Melting point | 178 °C (352 °F; 451 K) (decomposes)[1] |
| Boiling point | Decomposes |
| 3.5 g/100 ml (25 °C) | |
| Solubility | Insoluble in glacial acetic acid, diethyl ether |
| Vapor pressure | ~0 mmHg |
| Hazards | |
| Safety data sheet | Sigma-Aldrich (monohydrate) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Calcium gluconate is a calcium salt of gluconic acid. Among chemists, it is best known as an antidote for hydrofluoric acid exposure.
Contents
Properties
Physical
White powder, moderately soluble in cold water, readily soluble in hot water. It melts with decomposition around 178 °C, though some sources claim 120 °C.
Chemical
Heating causes calcium gluconate to decompose, liberating a large amount of carbon. This makes it a good candidate for a "Pharaoh's Serpent" experiment.
Availability and Uses
In some countries, such as the former Soviet bloc, calcium gluconate is available very cheaply in any drugstore in form of tablets or solutions. Tableted calcium gluconate, if it happens to be adulterated with chalk or something, can easily be purified by recrystallization through hot water. In other countries, a Russian pharmacy is a good place to start looking for it.
Its main medical use is as a calcium supplement. Chemists use it as an antidote for hydrofluoric acid poisoning, in forms of topical creamy gel or injections.
A 25% calcium gluconate aq. solution can be purchased from some farm and veterinary supplies, where it is intended for the treatment of milk fever. [2]
Projects
- Pharaoh's Snake
- Protect yourself while experimenting with hydrofluoric acid
Handling
Safety
Calcium gluconate is harmless. The medical grade salt is even safely edible as a dietary supplement.
Storage
Dry calcium gluconate or a solution thereof can be stored in any container under normal conditions.
Disposal
Calcium gluconate can be disposed of into the ground, trash or drain safely.
References
- ↑ Nilkantum; J.Sci.Technol.India; vol. 2; (1936); p. 39; Chem.Abstr.; (1938); p. 1403
- ↑ http://www.pbsanimalhealth.com/details/Calcium-Gluconate-23%25-Solution/13-86.html