Difference between revisions of "Heptane"
(Minor expansion) |
|||
| (6 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Chembox |
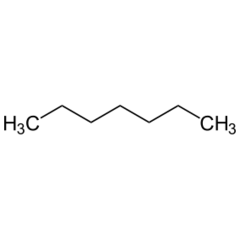
| − | '''Heptane''' or '''n-heptane''' is the straight-chain alkane with the chemical formula '''C<sub>7</sub>H<sub>16</sub>'''. | + | | Name = Heptane |
| + | | Reference = | ||
| + | | IUPACName = Heptane | ||
| + | | PIN = | ||
| + | | SystematicName = | ||
| + | | OtherNames = n-Heptane | ||
| + | <!-- Images --> | ||
| + | | ImageFile = Heptane bottle and sample.jpg | ||
| + | | ImageSize = 280 | ||
| + | | ImageAlt = | ||
| + | | ImageName = | ||
| + | | ImageCaption = n-Heptane bottle and sample from it | ||
| + | | ImageFile1 = | ||
| + | | ImageSize1 = | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageFile2 = | ||
| + | | ImageSize2 = | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = Heptane Structure.png | ||
| + | | ImageSizeL1 = 240 | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| + | | Section1 = {{Chembox Identifiers | ||
| + | | 3DMet = | ||
| + | | Abbreviations = | ||
| + | | SMILES = CCCCCCC | ||
| + | }} | ||
| + | | Section2 = {{Chembox Properties | ||
| + | | AtmosphericOHRateConstant = | ||
| + | | Appearance = Colorless liquid | ||
| + | | BoilingPt = | ||
| + | | BoilingPtC = 98.1 to 98.7 | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = | ||
| + | | Density = 0.6795 g/ml | ||
| + | | Formula = C<sub>7</sub>H<sub>16</sub> | ||
| + | | HenryConstant = 12 nmol Pa<sup>−1</sup> kg<sup>−1</sup> | ||
| + | | LogP = 4.274 | ||
| + | | MolarMass = 100.21 g/mol | ||
| + | | MeltingPt = | ||
| + | | MeltingPtC = −91.0 to −90.1 | ||
| + | | MeltingPt_ref = | ||
| + | | MeltingPt_notes = | ||
| + | | Odor = Gasoline-like | ||
| + | | pKa = | ||
| + | | pKb = | ||
| + | | Solubility = 0.0003% (20 °C) | ||
| + | | SolubleOther = Miscible with [[ethanol]] | ||
| + | | Solvent = | ||
| + | | VaporPressure = 5.33 kPa (at 20.0 °C) | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = | ||
| + | | CrystalStruct = | ||
| + | | MolShape = | ||
| + | }} | ||
| + | | Section4 = {{Chembox Thermochemistry | ||
| + | | DeltaGf = | ||
| + | | DeltaHc = −4.825–−4.809 kJ mol<sup>−1</sup> | ||
| + | | DeltaHf = −225.2–−223.6 kJ mol<sup>−1</sup> | ||
| + | | Entropy = 328.57 J K<sup>−1</sup> mol<sup>−1</sup> | ||
| + | | HeatCapacity = 224.64 J K<sup>−1</sup> mol<sup>−1</sup> | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = Non-explosive | ||
| + | | FrictionSens = | ||
| + | | DetonationV = | ||
| + | | REFactor = | ||
| + | }} | ||
| + | | Section6 = {{Chembox Hazards | ||
| + | | AutoignitionPt = 223.0 °C | ||
| + | | ExploLimits = 1.05–6.7% | ||
| + | | ExternalMSDS = [http://www.sciencelab.com/msds.php?msdsId=9924237 ScienceLab] | ||
| + | | FlashPt = −4.0 °C | ||
| + | | LD50 = 17,986 ppm (mouse, 2 hr) | ||
| + | | LC50 = | ||
| + | | MainHazards = Flammable, irritant | ||
| + | | NFPA-F = | ||
| + | | NFPA-H = | ||
| + | | NFPA-R = | ||
| + | | NFPA-S = | ||
| + | }} | ||
| + | | Section7 = {{Chembox Related | ||
| + | | OtherAnions = | ||
| + | | OtherCations = | ||
| + | | OtherFunction = | ||
| + | | OtherFunction_label = | ||
| + | | OtherCompounds = [[Hexane]]<br>[[Octane]] | ||
| + | }} | ||
| + | }} | ||
| + | '''Heptane''' or '''n-heptane''' is the straight-chain alkane with the chemical formula '''C<sub>7</sub>H<sub>16</sub>''', used as solvent. | ||
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| − | Heptane will burn when ignited in air. | + | Heptane will burn when ignited in air to release [[carbon dioxide]], [[water]] vapors and soot. |
| + | |||
| + | : C<sub>7</sub>H<sub>16</sub> + 11 O<sub>2</sub> → 7 CO<sub>2</sub> + 8 H<sub>2</sub>O | ||
===Physical=== | ===Physical=== | ||
| Line 13: | Line 124: | ||
Car starting fluids contain a mixture of heptane and [[diethyl ether]]. Due to the large difference between their boiling points, the mixture can be separated by first distilling the ether, and then the heptane. However, some formulas may also contain other isomers of heptane, making the extraction of the pure n-heptane complicated. | Car starting fluids contain a mixture of heptane and [[diethyl ether]]. Due to the large difference between their boiling points, the mixture can be separated by first distilling the ether, and then the heptane. However, some formulas may also contain other isomers of heptane, making the extraction of the pure n-heptane complicated. | ||
| + | |||
| + | Some barbecue lighter fluids tend to have a mixture of heptane and hexane, with a small addition of [[naphta]], while others can have relative pure heptane. | ||
==Preparation== | ==Preparation== | ||
| Line 20: | Line 133: | ||
*Identify aqueous bromine from iodine | *Identify aqueous bromine from iodine | ||
*Organic extractions | *Organic extractions | ||
| + | *Fuel | ||
==Handling== | ==Handling== | ||
| Line 39: | Line 153: | ||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
[[Category:Organic compounds]] | [[Category:Organic compounds]] | ||
| + | [[Category:Hydrocarbons]] | ||
[[Category:Alkanes]] | [[Category:Alkanes]] | ||
[[Category:Solvents]] | [[Category:Solvents]] | ||
[[Category:Nonpolar solvents]] | [[Category:Nonpolar solvents]] | ||
[[Category:Liquids]] | [[Category:Liquids]] | ||
Latest revision as of 23:20, 8 August 2020
 n-Heptane bottle and sample from it
| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Heptane
| |||
| Other names
n-Heptane
| |||
| Identifiers | |||
| Jmol-3D images | Image | ||
| |||
| Properties | |||
| C7H16 | |||
| Molar mass | 100.21 g/mol | ||
| Appearance | Colorless liquid | ||
| Odor | Gasoline-like | ||
| Density | 0.6795 g/ml | ||
| Melting point | −91.0 to −90.1 °C (−131.8 to −130.2 °F; 182.2 to 183.1 K) | ||
| Boiling point | 98.1 to 98.7 °C (208.6 to 209.7 °F; 371.2 to 371.8 K) | ||
| 0.0003% (20 °C) | |||
| Solubility | Miscible with ethanol | ||
| Vapor pressure | 5.33 kPa (at 20.0 °C) | ||
| Thermochemistry | |||
| Std molar
entropy (S |
328.57 J K−1 mol−1 | ||
| Std enthalpy of
formation (ΔfH |
−225.2–−223.6 kJ mol−1 | ||
| Hazards | |||
| Safety data sheet | ScienceLab | ||
| Flash point | −4.0 °C | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (Median dose)
|
17,986 ppm (mouse, 2 hr) | ||
| Related compounds | |||
| Related compounds
|
Hexane Octane | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Heptane or n-heptane is the straight-chain alkane with the chemical formula C7H16, used as solvent.
Contents
[hide]Properties
Chemical
Heptane will burn when ignited in air to release carbon dioxide, water vapors and soot.
- C7H16 + 11 O2 → 7 CO2 + 8 H2O
Physical
Heptane is a colorless liquid with a petroleum-like odor.
Availability
Certain types of lighter fluids contain heptane.
Car starting fluids contain a mixture of heptane and diethyl ether. Due to the large difference between their boiling points, the mixture can be separated by first distilling the ether, and then the heptane. However, some formulas may also contain other isomers of heptane, making the extraction of the pure n-heptane complicated.
Some barbecue lighter fluids tend to have a mixture of heptane and hexane, with a small addition of naphta, while others can have relative pure heptane.
Preparation
Heptane can be prepared by reducing heptane derivates. However it is generally much cheaper to simply buy the compound.
Projects
- Identify aqueous bromine from iodine
- Organic extractions
- Fuel
Handling
Safety
Heptane is flammable and it's vapors may be irritant if inhaled.
Storage
Heptane is best stored in closed bottles, kept in cold, dark and well ventilated places.
Disposal
Heptane can be safely burned in open air or in an incinerator.
References
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Hydrocarbons
- Alkanes
- Solvents
- Nonpolar solvents
- Liquids