Difference between revisions of "Butylated hydroxytoluene"
| Line 11: | Line 11: | ||
| ImageAlt = | | ImageAlt = | ||
| ImageName = | | ImageName = | ||
| − | | ImageFile1 = | + | | ImageFile1 = Butylated hydroxytoluene BHT structure.png |
| − | | ImageSize1 = | + | | ImageSize1 = 280 |
| ImageAlt1 = | | ImageAlt1 = | ||
| ImageName1 = | | ImageName1 = | ||
| + | | ImageCaption1 = Chemical tructure of BHT | ||
| ImageFile2 = | | ImageFile2 = | ||
| ImageSize2 = | | ImageSize2 = | ||
Revision as of 17:04, 30 June 2020
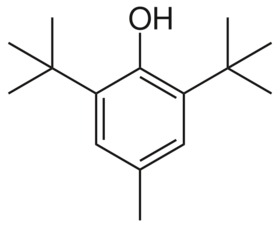 Chemical tructure of BHT
| |
| Names | |
|---|---|
| IUPAC name
2,6-Di-tert-butyl-4-methylphenol
| |
| Other names
2,6-Di-tert-butyl-p-cresol
3,5-(Dimethylethyl)-4-hydroxytoluene 3,5-Di-tert-butyl-4-hydroxytoluene 4-Methyl-2,6-di-tert-butyl phenol Additin RC 7110 AO-29 Avox BHT BHT DBPC Dibutylated hydroxytoluene E321 | |
| Properties | |
| C15H24O | |
| Molar mass | 220.356 g/mol |
| Appearance | White to yellow powder |
| Odor | Phenolic, aromatic |
| Density | 1.048 g/cm3 |
| Melting point | 70 °C (158 °F; 343 K) |
| Boiling point | 265 °C (509 °F; 538 K) |
| 1.1 mg/L (20 °C) | |
| Solubility | Soluble in acetone, benzene, cellosolve, cyclohexane, ethanol, heptane, hexane,isopropanol, methanol, methyl ethyl ketone, petroleum ether, toluene, vegetable oil Insoluble in propylene glycol |
| Vapor pressure | 0.01 mmHg (20°C) |
| Thermochemistry | |
| Std enthalpy of
formation (ΔfH |
-400 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 127 °C (261 °F; 400 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
2,000 mg/kg (rat, dermal) |
| Related compounds | |
| Related compounds
|
Phenol Butylated hydroxyanisole |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Butylated hydroxytoluene (BHT), also known as dibutylhydroxytoluene, is an organic compound, chemically a derivative of phenol, widely for its antioxidant properties, such as preservative for many reagents and other products.
Contents
Properties
Chemical
BHT acts as a synthetic structural analog of vitamin E, primarily acting as a terminating agent that suppresses autoxidation, a process whereby unsaturated (usually) organic compounds are attacked by atmospheric oxygen. BHT stops this autocatalytic reaction by converting peroxy radicals to hydroperoxides. It effects this function by donating a hydrogen atom:
- RO2• + ArOH → ROOH + ArO•
- RO2• + ArO• → nonradical products
where R is alkyl or aryl, and where ArOH is BHT or related phenolic antioxidants. Each BHT consumes two peroxy radicals.[1]
Physical
BHT is a white to yellowish crystalline solid insoluble in water, with a phenolic odor.
Availability
BHT is sold by chemical suppliers.
Phytoplankton, including the green algae Botryococcus braunii, as well as three different cyanobacteria (Cylindrospermopsis raciborskii, Microcystis aeruginosa and Oscillatoria sp.) are capable of producing minute amounts of BHT.
Preparation
Industrially, BHT is prepared by the reaction of p-cresol (4-methylphenol) with isobutylene (2-methylpropene), catalyzed by conc. sulfuric acid:
- CH3(C6H4)OH + 2 CH2=C(CH3)2 → ((CH3)3C)2CH3C6H2OH
Projects
- Antioxidant
- Reagent preservative
Handling
Safety
BHT is considered to posses low toxicity.
Storage
BHT should be kept in air-tight containers.
Disposal
No special disposal is required. Should be strongly diluted and poured down the drain.