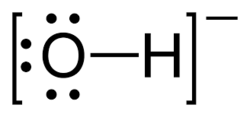Difference between revisions of "Hydroxide"
From Sciencemadness Wiki
| Line 1: | Line 1: | ||
{{stub}} | {{stub}} | ||
| − | [[File:OH drawing.png|thumb| | + | [[File:OH drawing.png|thumb|250x250px]] |
| − | Chemical hydroxides are very basic salts. As metal salts, they are usually very insoluble, but [[alkali]] hydroxides are very soluble, inexpensive [[ | + | Chemical '''hydroxides''' are very basic salts. As metal salts, they are usually very insoluble, but [[alkali]] hydroxides are very soluble, inexpensive [[base]]s. |
==Properties== | ==Properties== | ||
Revision as of 22:59, 2 January 2020
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
Chemical hydroxides are very basic salts. As metal salts, they are usually very insoluble, but alkali hydroxides are very soluble, inexpensive bases.
Contents
Properties
Chemical
Hydroxides are usually basic, and can be neutralized by acids (releasing the salt and water).
Physical
Alkali hydroxides are very soluble and very basic, while other hydroxides are usually only slightly basic and insoluble.
Production
Addition of an alkaline metal oxide to water gives hydroxide.