Difference between revisions of "Benzophenone"
m (→Chemical) |
|||
| Line 5: | Line 5: | ||
| IUPACName = Diphenylmethanone | | IUPACName = Diphenylmethanone | ||
| PIN = | | PIN = | ||
| − | | OtherNames = Benzophenone<br> Diphenyl ketone<br> Benzoylbenzene<br> Benzoylphenyl<br> Diphenylmethanone | + | | OtherNames = Benzophenone<br>Diphenyl ketone<br>Benzoylbenzene<br>Benzoylphenyl<br>Diphenylmethanone |
<!-- Images --> | <!-- Images --> | ||
| ImageFile = Benzophenone.png | | ImageFile = Benzophenone.png | ||
| Line 53: | Line 53: | ||
| BoilingPt_ref = | | BoilingPt_ref = | ||
| BoilingPt_notes = | | BoilingPt_notes = | ||
| − | | Density = 1.11 | + | | Density = 1.11 g/cm<sup>3</sup> (20 °C) |
| Formula = '''[[Carbon|C]]'''<sub>13</sub>'''[[Hydrogen|H]]'''<sub>10</sub>'''[[Oxygen|O]]''' | | Formula = '''[[Carbon|C]]'''<sub>13</sub>'''[[Hydrogen|H]]'''<sub>10</sub>'''[[Oxygen|O]]''' | ||
| HenryConstant = | | HenryConstant = | ||
| − | | LogP = | + | | LogP = 3.18 |
| − | | MolarMass = 182.22 | + | | MolarMass = 182.22 g/mol |
| MeltingPt = | | MeltingPt = | ||
| MeltingPtC = 48.5 | | MeltingPtC = 48.5 | ||
| MeltingPt_ref = | | MeltingPt_ref = | ||
| MeltingPt_notes = | | MeltingPt_notes = | ||
| + | | Odor = Geranium-like | ||
| pKa = | | pKa = | ||
| pKb = | | pKb = | ||
| − | | | + | | Solubility = 0.0137 g/100 ml (at 25 °C) |
| − | | | + | | SolubleOther = Soluble in glacial [[acetic acid]], [[acetone]], [[benzene]], [[carbon disulfide]], [[carbon tetrachloride|CCl<sub>4</sub>]], [[methanol]] |
| − | | | + | | Solubility1 = 16.6 g/100 ml |
| − | | | + | | Solvent1 = diethyl ether |
| − | | VaporPressure = | + | | Solubility2 = 13.3 g/100 ml |
| + | | Solvent2 = ethanol | ||
| + | | VaporPressure = 1.93·10<sup>-3</sup> mm Hg at 25 °C | ||
}} | }} | ||
| Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| Line 89: | Line 92: | ||
}} | }} | ||
| Section6 = {{Chembox Hazards | | Section6 = {{Chembox Hazards | ||
| − | | AutoignitionPt = | + | | AutoignitionPt = 650 °C (1202 °F; 923 K) |
| ExploLimits = | | ExploLimits = | ||
| − | | ExternalMSDS = | + | | ExternalMSDS = [] |
| − | | FlashPt = | + | | FlashPt = 110 °C (230 °F; 383 K) |
| LD50 = | | LD50 = | ||
| LC50 = | | LC50 = | ||
| − | | MainHazards = | + | | MainHazards = Irritant |
| NFPA-F = | | NFPA-F = | ||
| NFPA-H = | | NFPA-H = | ||
| Line 106: | Line 109: | ||
| OtherFunction = | | OtherFunction = | ||
| OtherFunction_label = | | OtherFunction_label = | ||
| − | | OtherCompounds = | + | | OtherCompounds = [[Acetophenone]] |
}} | }} | ||
}} | }} | ||
| Line 116: | Line 119: | ||
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| − | Benzophenone can be reduced by [[sodium]] metal to produce the intensely blue | + | Benzophenone can be reduced by [[sodium]] metal to produce the intensely blue colored benzophenone ketyl radical. This is highly reactive towards [[water]] and [[oxygen]] so it can be used as an indicator of solvent dryness. |
Benzophenone is a strong UV absorber, owing to cross-conjugation. This results in its use as a UV photoinitiator in some UV curing resins. Derivatives of benzophenone are often used in sunscreens and as an additive to prevent the UV-induced degradation of polymers. | Benzophenone is a strong UV absorber, owing to cross-conjugation. This results in its use as a UV photoinitiator in some UV curing resins. Derivatives of benzophenone are often used in sunscreens and as an additive to prevent the UV-induced degradation of polymers. | ||
===Physical=== | ===Physical=== | ||
| − | + | Benzophenone is a white solid, with a characteristic smell, practically insoluble in water, but more soluble in organic solvents. | |
==Availability== | ==Availability== | ||
| − | + | Benzophenone is sold by lab suppliers. Can also be purchased from eBay. | |
==Preparation== | ==Preparation== | ||
| − | + | Benzophenone is produced by the copper-catalyzed oxidation of diphenylmethane with oxygen from air. | |
==Projects== | ==Projects== | ||
| − | + | *Photosensitizer in photochemistry | |
| + | *Water indicator in air-free techniques | ||
==Handling== | ==Handling== | ||
| − | |||
===Safety=== | ===Safety=== | ||
| + | Benzophenone is considered safe, but it may be irritant. | ||
===Storage=== | ===Storage=== | ||
| + | In sealed bottles. | ||
===Disposal=== | ===Disposal=== | ||
| + | Can be neutralized by oxidizing it with an oxidizing solution/mixture. | ||
==References== | ==References== | ||
<references/> | <references/> | ||
===Relevant Sciencemadness threads=== | ===Relevant Sciencemadness threads=== | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=42529 Preparation of Benzophenone] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=11208 benzophenone synthesis] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=20540 Lewis acid for benzophenone synthesis with benzoyl bromide] | ||
Revision as of 20:39, 3 December 2018
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
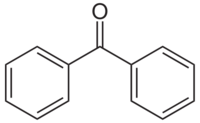
| |
| Names | |
|---|---|
| IUPAC name
Diphenylmethanone
| |
| Other names
Benzophenone
Diphenyl ketone Benzoylbenzene Benzoylphenyl Diphenylmethanone | |
| Identifiers | |
| 119-61-9 | |
| Jmol-3D images | Image |
| |
| Properties | |
| C13H10O | |
| Molar mass | 182.22 g/mol |
| Appearance | White solid |
| Odor | Geranium-like |
| Density | 1.11 g/cm3 (20 °C) |
| Melting point | 48.5 °C (119.3 °F; 321.6 K) |
| Boiling point | 305.4 °C (581.7 °F; 578.5 K) |
| 0.0137 g/100 ml (at 25 °C) | |
| Solubility | Soluble in glacial acetic acid, acetone, benzene, carbon disulfide, CCl4, methanol |
| Solubility in diethyl ether | 16.6 g/100 ml |
| Solubility in ethanol | 13.3 g/100 ml |
| Vapor pressure | 1.93·10-3 mm Hg at 25 °C |
| Hazards | |
| Safety data sheet | [] |
| Flash point | 110 °C (230 °F; 383 K) |
| Related compounds | |
| Related compounds
|
Acetophenone |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Benzophenone is the simplest di-aryl ketone.
It is often used in drying solvents together with sodium metal.
Contents
Properties
Chemical
Benzophenone can be reduced by sodium metal to produce the intensely blue colored benzophenone ketyl radical. This is highly reactive towards water and oxygen so it can be used as an indicator of solvent dryness.
Benzophenone is a strong UV absorber, owing to cross-conjugation. This results in its use as a UV photoinitiator in some UV curing resins. Derivatives of benzophenone are often used in sunscreens and as an additive to prevent the UV-induced degradation of polymers.
Physical
Benzophenone is a white solid, with a characteristic smell, practically insoluble in water, but more soluble in organic solvents.
Availability
Benzophenone is sold by lab suppliers. Can also be purchased from eBay.
Preparation
Benzophenone is produced by the copper-catalyzed oxidation of diphenylmethane with oxygen from air.
Projects
- Photosensitizer in photochemistry
- Water indicator in air-free techniques
Handling
Safety
Benzophenone is considered safe, but it may be irritant.
Storage
In sealed bottles.
Disposal
Can be neutralized by oxidizing it with an oxidizing solution/mixture.