| Pages:
1
2
3 |
CyrusGrey
Hazard to Others
  
Posts: 123
Registered: 20-1-2007
Location: USA
Member Is Offline
Mood: Oooh! Shiny!
|
|
Unknown copper compound
Several months ago I used HCl with a little CuCl to dissolve the copper cladding off of some graphite welding rods. Well that had been sitting with
the now stripped rods in it until now. I took the rods out and thought that I would try and do something useful with the liquid left over, it was a
dark green at this time. I put a copper pipe in it that had been corroded with HCl fumes. After several days I ended up with a very dark brown liquid
that I cant identify (It looks black, almost so even in a test tube).
I did the following tests on it:
Dilution with distilled water yields a white precipitate (CuCl?) and the solution has a very light greenish tint
Heating the liquid seems to do nothing, then when its diluted it gives the white precipitate but the brown remains
Adding ascorbic acid makes the brown color much lighter, heating this makes it darker again but not to the original darkness
Adding KNO3 seems to do nothing but then when this is heated it bubbles for a moment, releasing a brown gas the same color as the solution (NO2?) and
the solution turns the dark green color of the solution before I added the pipe, when this is diluted it becomes bluish-green
Heating a small amount of the liquid until dryness yields a black solid, which further dehydrates to a tan color, adding water back makes it green
What is this stuff? I'm still a bit of a chemistry newbe, but I've looked around and havent found any brown soluble copper compounds with the stuff I
started out with.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
http://www.sciencemadness.org/talk/viewthread.php?tid=9882
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
CyrusGrey:
Yes, that looks talks and walks very much like what is suspected to be a Cu+ - Cu2+ - Cl- complex discussed on that thread, no doubt about it.
Interestingly enough, you seem to have actually isolated the compound in "pure" (i.e. crystalline) form. I've been meaning to do that too but haven't
gotten round to it yet. It would confirm what I've suspected for some time: the complex is remarkably stable.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
| Quote: | Originally posted by CyrusGrey
Dilution with distilled water yields a white precipitate (CuCl?) and the solution has a very light greenish tint |
Yes, this is CuCl, contaminated with some blue copper(II) compound. This contamination makes it appear exceptionally white. Any attempt to isolate and
dry the white material makes it green or even brown, due to partial oxidation.
| Quote: | | Heating the liquid seems to do nothing, then when its diluted it gives the white precipitate but the brown remains |
This is understandable. There is an equilibrium between CuCl and the complex CuCl2(-) and a dark brown copper(I)/copper(II) complex. Your solution
contains excess copper(I). On dilution, the complex dissociates into CuCl and chloride. The CuCl precipitates.
I did the reverse. I have some CuCl (commercial sample). This dissolves in conc. HCl very easily, while it is insoluble in water. The solution is
almost colorless, but when air is allowed to reach the solution, then it quickly turns deep brown. When much more air is allowed to act upon the
solution, then it turns lighter again and it becomes green/yellow, due to formation of CuCl4(2-).
| Quote: | | Adding ascorbic acid makes the brown color much lighter, heating this makes it darker again but not to the original darkness. |
Understandable. The ascorbic acid reduces any copper(II) in solution, giving less of the copper(I)/copper(II) complex and making more colorless
CuCl2(-) in solution.
| Quote: | | Adding KNO3 seems to do nothing but then when this is heated it bubbles for a moment, releasing a brown gas the same color as the solution (NO2?) and
the solution turns the dark green color of the solution before I added the pipe, when this is diluted it becomes bluish-green |
The nitrate ion oxidizes any copper(I) in solution, itself being reduced to NO2/NO. This makes the solution green/yellow, because only copper(II)
remains in solution. However, the solution becomes dark green, because another new complex plays a role in this. Copper(II), chloride and NO2 form a
dark blue complex, which with the yellow/green of the CuCl4(2-) gives a dark green color.
The copper(II)/chloride/NO2 complex also is not a well-known thing. I have written a web-page on that as well:
http://woelen.scheikunde.net/science/chem/riddles/copper+nit...
| Quote: | Heating a small amount of the liquid until dryness yields a black solid, which further dehydrates to a tan color, adding water back makes it green
What is this stuff? I'm still a bit of a chemistry newbe, but I've looked around and havent found any brown soluble copper compounds with the stuff I
started out with. |
The tan material is mainly CuCl2, anhydrous. CuCl2.2H2O is a cyan/blue compound, but the anhydrous material is dark brown, when in the form of big
crystals, when it is finely powdered it is much lighter brown. On addition of some water, it becomes green, due to complex formation.
-----------------------------------------------------------------
Copper is my favorite element. It has such a rich aqueous chemistry and there really is a lot to be discovered and explained. Even modern textbooks do
not mention all these remarkable complexes, while they can be obtained with such common and simple reagents. Maybe modern chemists seem to find this
kind of chemistry too common, possibly overlooking some interesting aspects of it.
[Edited on 6-2-08 by woelen]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Hi Woelen:
I saw your page on the Cu2+/NO2 complex, obtained by adding NaNO2 to Cu2+ solutions.
But what you (and CyrusGrey) describe in relation to NO3 (-) does not sit well with my own observations.
At first, I've been using mixtures of Cu(NO3)2 (0.4 M) and NaCl, to simulate CuCl2 (I didn't have any CuCl2 at the time) and although I've always
obtained a dark green solution as well as quite a bit of CuCl precipitating out of the solution when reacting it with copper metal.
Later I did use CuCl2 and the same dark green appeared: in fact the system Cu(NO3)2 = 0.4 M + NaCl = 0.8 M and the system CuCl2 = 0.4 M react almost
in the same way, with Cu or even with sodium metabisulfite as a reducing agent: I always get a dark green solution and precipitate of CuCl. In
Cu(NO3)2 solutions of higher NaCl concentration, the CuCl forms also but on cooling or dilution of the solution. Never have I noticed significant
amounts of NO/NO2 developing using these copper nitrate solutions, even at BP.
Is it possible that the dark green solution is merely due to concentration and that on much more prolonged boiling the solution would darken
further and further?
An experiment very similar to (and inspired by) your reduction by means of Zn experiment seems to confirm this. Here I've dissolved a pinch of CuCl2
in a test tube in a few ml of 32 w% HCl and divided it into equal amounts over two test tubes. To the first a small ball of copper wire was added and
both tubes were properly stoppered. The colour of the copper wire containing solution gradually darkens over time. From a slight darkening at first,
the solution, now about three days into the experiment, has turned almost black but is still transparent and without any precipitate (Cl- does indeed
complex CuCl into CuCl2 (-) very well).
Interestingly, up to and including yesterday, the bottom of the test tube (where the copper wire rests) sometimes cleared up completely, presumably
where Cu2+ had been reduced to Cu+ completely and without agitation two layers formed.
The shades of green (and now brown) in this experiment are different from the ones obtained with Cu(NO3)2 but then I never used such high
concentrations of Cl- and H3O+ before either.
It now remains to be seen if in the coming days the solution will actually clear when all the Cu2+ has been reduced to Cu+ and the presumed Cu+ - Cu2+
- Cl- complex can no longer exist.
You're right about copper chemistry being very interesting and perhaps parts of it being a little overlooked. And this complex has got me thoroughly
hooked, so much so that I'm thinking of carrying out another experiment.
It would be interesting IMHO to try and correlate the colour of the solution to the Cu2+/Cu+ molar ratio.
Using a larger version of the test tube experiment and using small, thin copper plates or copper foil as copper substrate, it should be possible to
determine the Cu2+/Cu+ molar ratio (as well as the total amount of copper in solution) by means of the weight loss of the copper substrate and
correlate this with the colour of the solution. Even better of course would be to quantify the colour by means of UV/VIS absorption spectrometry...
Have you ever contemplated this?
[Edited on 7-2-2008 by blogfast25]
[Edited on 7-2-2008 by blogfast25]
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Blogfast25, one experiment which you could do is the following:
Dissolve some Cu(NO3)2.xH2O in dilute sulphuric acid and add a small pinch of NaCl. Only a small amount of chloride must be added.
Take another test tube with Cu(NO3)2.xH2O and heat, such that you get some brown NO2 gas.
Pour the acidic solution with copper nitrate, a little chloride and the dilute sulphuric acid into the test tube with the NO2. If you do this, I
expect that you will get the deep blue complex.
Of course, if you have another easy way of making NO2, then you can use that. I just gave the 'recipe' above, in order to minimize the number of
different reagents needed (only copper nitrate, sodium chloride and dilute sulphuric acid).
In your description I read that you did the copper nitrate + NaCl experiment, but not at high acid concentration. Try dissolving some copper nitrate
in conc. HCl and then heat. I really expect formation of some orange/brown gas.
The molar ratio experiment sounds interesting, and I am willing to try some of that, but it is really difficult. Solutions of copper (I) in conc. HCl
absorb oxygen from air EXTREMELY easily. That really is a big problem when you want to do quantitative work. I'll think about this and see what I can
do. A collaboration between different people over here is a good thing and possibly could result in new interesting results  . .
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Oh, regards the Cu2+/NO2 complex, I can produce that very easily, I'm sure. I make my Cu(NO3)2 by dissolving copper scrap into 35 w% HNO3, so plenty
of NOx generated there (LOL).
But I'm simply not convinced the green colour is a mix between CuCl4 (2-) and the NO2 complex: how otherwise to explain it does also appear (in my
conditions) with CuCl2, even with CuCl2 and metabisulfite? An alternative explanation is that concentration of the Cu+/Cu2+/Cl- complex, [CuCl4
(2-)] and the pH affect the colour of the solution. For the most part, I have worked close to pH = 7. Adding a large excess of HCl prevents CuCl
dropping out of course (but my original purpose was to harvest the CuCl).
| Quote: | Originally posted by woelen
The molar ratio experiment sounds interesting, and I am willing to try some of that, but it is really difficult. Solutions of copper (I) in conc. HCl
absorb oxygen from air EXTREMELY easily. That really is a big problem when you want to do quantitative work. I'll think about this and see what I can
do. A collaboration between different people over here is a good thing and possibly could result in new interesting results  . . |
Careful stoppering, allowing as little headspace between liquid and air as possible and a few other simple measures should be quite effective against
oxygen contamination, IMHO.
Another trick I used to use when titrating Ti3+ solutions (with Fe3+, if I recall well) is that of a CO2 blanket: adding a small amount of
bicarbonate causes CO2 to form (in acid conditions of course), preventing oxygen from entering the solution. This way you can titrate the highly
oxygen sensitive Ti3+ with completely normal equipment (conical flask + burette). Very effective.
Alternatively, one could set up a range of tests, all containing the same amount of the same starting solution (of known [Cu2+]) and all containing an
accurately weighed (and more or less equal) amount of copper metal (say wire). The solutions would then all be allowed to run 1, 2, 3, ..., n
days and each day the amount of digested copper could be determined, after photographing the solution (or UV/VIS). From there determining the total
amount of Cu in solution and the ratio Cu2+/Cu+ could be estimated, without oxygen interference. Copper is heavy, so it provides good gravimetric
leverage.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
| Quote: | | But I'm simply not convinced the green colour is a mix between CuCl4 (2-) and the NO2 complex |
Yep, I've been
thinking about this and did another simple test with the blue copper complex with NO2. You're right, the green color is not a mix with the
NO2-complex. The NO2-complex cannot even exist under those conditions, it only exists at moderately low concentration of chloride.
You raised another interesting point. Time to go back to the lab and try the copper(II)/HCl/KNO3 mix myself  . .
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
In my experience, "dilute" solutions of the brown complex appear dark green. This can be seen by making a strong CuCl2 solution and adding a reducing
agent (e.g., Cu), maybe with a little acid. The color goes from green (CuCl4(2-)) to dark green to brown.
Tim
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Back from the lab 
I now have come to the conclusion that the dark green color is due only to increase of concentration. The nitrate does not really matter.
I did the following experiment:
- dissolve 100 mg CuCl2.2H2O in 3 ml of conc. HCl (30%). This results in a yellow/green solution. On heating, this solution becomes a little bit more
yellow, less green.
- dissolve 500 mg of CuCl2.2H2O in 1 ml of conc. HCl (30%). This results in a very dark green solution. On heating, this solution becomes very dark
brown. On cooling down, it becomes dark green again.
So, it all is a matter of concentration, but the color also depends on temperature.
To both solutions, I added some NaNO3. This does not result in a drastic change. On heating, the color again changes, it becomes brown, and some gas
is produced (strong smell of Cl2, but also ONCl, which has a peculair odour). The color after heating and cooling down again is somewhat different
than without the nitrate added, but that can be explained. ONCl has a strong orange color in solution, this affects the total observed color.
-----------------------------------------------------------
Finally, I did the following experiment:
- take 1 ml of HNO3 (52%)
- take 2 ml of HCl (30%)
- mix both chemicals
- add 1 spatula full of fine copper powder (< 50 um particle size)
- heat
On heating, the liquid quickly turns dark green, but soon, it becomes totally black and opaque. Probably the copper (I)/copper(II)/chloride complex is
formed. I added another 2 ml of the HNO3/HCl mix. This did not really change things. It only became a little bit lighter and deep green again. On
heating, again it became totally black.
Then I put aside the black, still hot solution. After half a minute or so, suddenly, a very violent reaction started. The material in the test tube
was spewn out in an eruption of brown gas and a dark green liquid. It was quite scary  . I had the test tube in a sink, and inside the sink, I could see a brown/yellow color of NO2. A lot of this gas was produced. I ran away
from the lab not wanting to breathe such a high concentration of this gas, such that one can see it . I had the test tube in a sink, and inside the sink, I could see a brown/yellow color of NO2. A lot of this gas was produced. I ran away
from the lab not wanting to breathe such a high concentration of this gas, such that one can see it  . Here endeth my experiment . Here endeth my experiment  . .
More will follow, but now I know about the possibility of violent runaway, I will have to be more careful. I think that the dark black material,
dissolved in the HNO3/HCl mix suddenly is oxidized at once. I saw the reaction starting near the surface and within two seconds a 'reaction front
wave' propagated through the liquid. I must not think of what could have happened if this was 50 ml or so. Now I only had 5 ml of liquid.
|
|
|
CyrusGrey
Hazard to Others
  
Posts: 123
Registered: 20-1-2007
Location: USA
Member Is Offline
Mood: Oooh! Shiny!
|
|
Woelen: I also observed this reaction when I added the KNO3. I only used maybe 50mg of KNO3 though so it didnt spew out so much NO2. It seems to turn
from brown to green with no change in concentration on addition of nitrates or heating in air. I'm going to try some other oxidisers and see if I get
the same reaction.
The copper in my beaker of the brown solution continues to dissolve, but only slowly now. There is also a layer of white CuCl precipitate in the
bottom now. Also at the interface between the air and the solution where the copper pipe comes out of it there is a green and white solid covering it.
|
|
|
CyrusGrey
Hazard to Others
  
Posts: 123
Registered: 20-1-2007
Location: USA
Member Is Offline
Mood: Oooh! Shiny!
|
|
I put some of the brown solution into three test tubes, about 2mL each.
In the first one I put about 50-100mg of potassium permaganate, it immediatly turned dark green, and the test tube was warm.
In the second I put about 150mg of Ca(OCl)2. It immediatly started bubbling and frothed up. The smell of chlorine gas was strong, and the test tube
was again very warm for the amount of solution.
In the third I put a few drops of 17-18% H2O2 solution. I got a very nice seperation of the solution into two layers. The top was the dark green and
the bottom was brown. I gently shook the test tube a little, and the layers just did not want to mix. I added about a dozen more drops and I got three
layers of solution, the topmost one was a light blue-green. There was a perfectly clear boundry between each layer. Again I shook the test tube and
the brown layer slowly dissapeared after about 30 seconds of shaking. The blue-green and dark green layers didnt want to mix either. During this time
the solution was bubbling slightly and the tube was warm.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
As I reported here,
http://www.sciencemadness.org/talk/viewthread.php?tid=9831
copper(I) solutions can act as a reasonable oxidation-absorber, acting catalytically between anything oxidizing (chlorate, chlorine, H2O2, etc.) and
anything reducing (copper(0), etc.). Excess oxidizer will result in an excess of its active species (HClO3, O2, etc.).
Tim
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
CyrusGrey, the reaction with H2O2 indeed is very nice. I made a picture of that, quite some time ago already:
[img]http://woelen.scheikunde.net/science/chem/riddles/copperI+copperII/exp0004/exp0004-1.jpg[/img]
A nice multilayer system is formed, which only very slowly degrades.
EDIT: ????
please copy the url between the [img] tags. I don't understand why the image is not included in the message itself.
[Edited on 8-2-08 by woelen]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yep, no doubt the colour of these complexes depends on concentration, other spectator ions (such as H3O+) and probably temperature. I was reading up
on the VIS absorption spectra of coordination complexes in Shriver and Atkins' 'Inorganic Chemistry' and the quantumchemical explanation of these
spectra is quite something. I'll need to do some re-studying to come to grips with that stuff again!
My test tube experiment (HCl, CuCl2 and Cu) now looks completely black and doesn't seem to change anymore (but it's hard to tell). There's plenty
copper left. I'll let this run on to see if eventually this solution clears or whether the Cu2+ - Cu+ - Cl- (my tentative formula is Cu2Cl4
(-) ) resists further reduction in these conditions.
For the Cu+/Cu2+ molar ratio experiment I now have a tentative design. I'll be using an old jam jar of more or less cubical shape (clear glass), about
100 - 200 ml volume, loaded with a CuCl2/HCl solution and a few clean (cleaned with HNO3) pieces of copper gas tubing. It'll be easy to remove (and
reload them after weighing) the copper pieces, without too much oxygen contamination (although some is inevitable).
I haven't decided on the CuCl2 concentration yet. A 0.4 M solution will discolour quickly and the colour will probably 'saturate' quite fast. A lower
concentration will perhaps allow a larger range of colours but the experiment will take longer.
Another thing that would be interesting to do is to check what kind of precipitate is obtained when at various degrees of reduction when you drop some
of the solution into dilute NaOH. You [Woelen] obtained a brownish yellow but I've obtained a whole plethora of colours (unfortunately not in
systematically controlled conditions), including this kind of green, more kakhish green, brown and
occasionally even blue.
In one case I diluted the solution (after removing the copper) quite a bit and obtained the same kind of precipitate from the more concentrated and
from the diluted solution. This seems to suggest the complex is indeed one well-defined species.
It's puzzling nonetheless...
[Edited on 8-2-2008 by blogfast25]
[Edited on 8-2-2008 by blogfast25]
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Woelen, I was interested by the riddle proposed on your website. I think I may have figured out why the complex is so short lived and changes color.
NO2 has linkage isomerism; It can coordinate with its oxygen lone pair (nitrito ligand) or its nitrogen lone pair (nitro ligand). For example there
are two [Co(NO2)(NH3)5]++ complexes. The nitrito compound is red; the nitro compound is yellow. Over time the unstable nitrito complex forms the
yellow nitro complex. It seems very possible that you are observing the same effect with a different complex.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Complexation of CuCl - Estimate of Kf
Assume the following system in equilibrium:
An aqueous solution of solid CuCl, a small amount of a strong, monoprotonic acid HA (but not HCl) to suppress OH(-) (so, [OH(-)] = 0), and a
concentration c (mol/L) of a strongly dissociated chloride, MCl.
MCl --> M(+) + Cl(-) [M(+)] = c (eq. 1)
HA + H2O --> H3O(+) + A(-) [H3O(+)] = [A(-)] (eq. 2)
CuCl <--> Cu(+) + Cl(-) Ks = [Cu(+)] . [Cl(-)] (eq. 3)
CuCl + Cl(-) <--> CuCl2 (-) Kf = [CuCl2 (-)] / [Cl(-)] (eq. 4)
Neutrality:
[H3O(+)] + [M(+)] + [Cu(+)] = [CuCl2 (-)] + [Cl(-)] + [A(-)] (eq. 5)
From these equations [CuCl2 (-)] and the other concentrations can be derived:
[CuCl2 (-)] = Kf .{[ c + √(c^2 + 4.(1+Kf).Ks) ] / [2.(1+Kf)]} (eq. 6)
A few special cases:
a. c = 0, then [CuCl2 (-)] = Kf. √[Ks/(1+Kf)] (eq. 7)
[Cl(-)] = √[Ks/(1+Kf)] (eq. 8)
and [Cu(+)] = Ks/[Cl(-)]
b. c large and Ks << Kf
[CuCl2 (-)] ≈ c.Kf/(1+Kf) (eq. 9)
Eq. 6 is a positively sloped, continuous function with eq. 7 as intercept and tending to eq. 9 asymptotically (a practical determination of Kf would
probably boil down to determining the slope of this asymptote).
An estimate of Kf can be obtained as follows. Ks = 1.7 10^-7 (mol/L)^2 and from Wiki the solubility of CuCl in water at 20 DC is S = 0.0062 g/100 mL
or 6.3 10^-4 mol/L. Note that this value of S does not specify method or pH and is a single data point.
From S = {[CuCl2 (-)] + [Cl(-)]} and eq. 7 and 8, Kf can then be estimated:
Kf (est.) = 1.34 and pKf (est.) = - 0.13
Although this is a very rough estimate, it sits quite well with the observation that in concentrated solutions of HCl or NaCl, CuCl is quite soluble.
For 2 M NaCl, [CuCl2 (-)] ≈ 1.15 M for example.
++++++
Unfortunately this also shows (by another route) that the proposed Cu(2+)/Cu(+) molar ratio experiment is unlikely to shed much light on the
composition of the unknown Cu(2+)/Cu(+)/Cl(-) complex, as in the presence of excess HCl or NaCl, much of the Cu(+) is present as CuCl2 (-).
In the absence of excess HCl or NaCl, the overall Cl to Cu ratio remains 2, no matter how far the reduction has been carried, which could tentatively
suggest Cu2Cl4 (-): perhaps {Cl(-) - Cu(+) - Cu(2+) - 3 Cl(-)}(-) ...???
[Edited on 10-2-2008 by blogfast25]
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Some time ago I found a text on Internet, which also was about a dark copper(I)/copper(II) complex, but I can't find it back anymore  (stupid me, that I did not save the link). (stupid me, that I did not save the link).
The text made mention of a neutral Cl-Cu-Cl-Cu-Cl complex, with all chlorines in formal oxidation state -1, and both coppers in oxidation state +1.5
(both coppers were mentioned as being equivalent in the complex). The middle Cl is bound in a special way, such that it forms a bridge between the two
copper-atoms. The bond also is fairly weak/labile, such that it easily is broken down (e.g. by oxidizers, but also by dilution).
I'll do my best to find it back again. I really hope to find back the text. The text already was somewhat older (IIRC from the 1960's), it was a
scanned document.
I can imagine such a structure. A resonance structure over a larger length may indeed explain its strong color.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Possibly... Cl- is a Lewis base and can form bridges between various metal centres.
My test tube experiment has used up all copper (I've now added some more) it seems to go darker still.
But I haven't seen a great deal of evidence that this complex breaks down on dilution...
[Edited on 10-2-2008 by blogfast25]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
It does. Try diluting 10x or more. You'll see a blue solution with white precipitate, CuCl, suspended.
Tim
|
|
|
CyrusGrey
Hazard to Others
  
Posts: 123
Registered: 20-1-2007
Location: USA
Member Is Offline
Mood: Oooh! Shiny!
|
|
Ah, Woelen, your photography skills are better than mine then. Heres the photo of my mixture:
[Edited on 11-2-2008 by CyrusGrey]
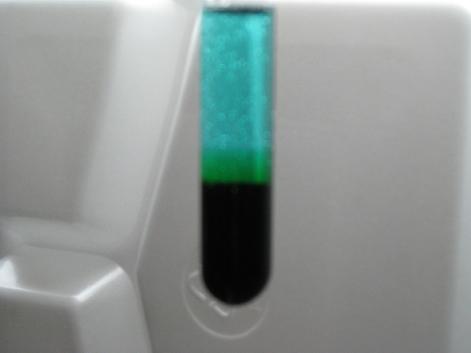
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Today I accidentally found out an easy way to make what I'm pretty sure is CuCl (yellow insoluble copper compound). I had a pretty concentrated NaCl
solution with copper electrodes supplied by an old printer DC supply. The anode had a fairly high surface area (a spiral of about 6 feet of wire).
When the cathode (3 4inch sections of copper pipe wired together) was fully submerged there was very little visible action at all even with the full
20 volts on it for an hour. The interesting part is when the cathode was mostly pulled out of the solution with just a few millimeters submerged it
bubbled strongly and within minutes there was a mass of very fine yellow precipitate floating at the surface. The supply got warm indicating there was
actually current (maybe 3 amps) while there was little before. More info when I have a chance. Any idea what exactly is going on here?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by 12AX7
It does. Try diluting 10x or more. You'll see a blue solution with white precipitate, CuCl, suspended.
Tim |
Well, as luck would have it, yesterday I had to stop my test tube experiment because the cork stopper had suffered too much from the HCl fumes. By
then the solution was basically black.
I diluted it 1:1 first and that caused the black to shift to dark brown. No precipitate formed. Not after standing overnight either.
I then diluted that solution another 1:1 time (by then it was 1 volume original : 3 volumes deionised water). It shifted to that characteristic green
(distinctly different from the cyan/greenish of CuCl2). Still no precipitate formed. Not after standing overnight either.
That the complex hydrolyses in high dilution is very well possible but I think in most cases the precipitate is causes by hydrolysis of CuCl2 (-). If
your solution contains much free Cl(-) (from HCl or NaCl) then much of the Cu(+) will be complexed as CuCl2 (-) and diluting it pushes CuCl + Cl(-)
<---> CuCl2 (-) to the left.
The solution used here was of course in 32 w% HCl, so even at 1:4 dilution it's still about 2.5 M in HCl, perhaps strong enough to keep Cu(+)
complexed.
Interestingly, adding some of the (1:4) diluted solution to the original control (CuCl2 in strong HCl) caused the control to shift to a colour
slightly darker (green) than the two separate solutions. Maybe some more complex is being formed from the excess Cu(2+) in the control and some Cu(+)
in the treated solution.
Having read your post, I've just poured a fairly small amount of the (1:4) diluted solution into a relatively large volume of dionised water and I see
no CuCl suspended at all. The colour seems simply diluted.
I'll check what kind of precipitate these solutions yield with diluted NaOH.
I'm setting up three more test tubes: one with pure CuCl2, one with CuCl2 + HCl, one with CuCl2 + NaCl. All in comparable concentrations, all with Cu
present.
497:
CuCl is actually white, not yellow. It's possible you're forming CuCl and that it's hydrolysing to Cu2O, which when very fresh and finely divided
would look yellowish. Can you check the pH in the area where the yellow is appearing?
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
@497: As mentioned above, the yellow material most likely is Cu2O, finely divided. I know this yellow/ochre material you get. You also get it by
simply taking two electricity wires and using that as electrode set with a 9V power supply. For me, it is not really clear why Cu2O is formed, even in
a neutral solution of NaCl. I would expect formation of CuCl2(-) in the concentrated solution of NaCl. Yes, welcome to the complex world of copper
chemistry  . .
@CyrusGrey: That picture you show resembles what I have, the main difference being that you start with an already very dark solution, and I start with
a solution, which only contains a small amount of the dark complex. In my situation you can see that there is a dark band, which is caused by the
Cu(2+), formed just below the H2O2-layer, which reacts with Cu(+)/Cl(-) in the lower part of the liquid.
@blogfast25: The CuCl-precipitate is obtained if you dissolve a lot of copper metal (as much as possible) in a mix of equal volumes of HCl (30%) and
H2O2(20%). Add a lot of copper wire (the thin wires from electricity cord is best) to conc. HCl and then slowly with stirring add H2O2 (20%) in small
steps. Each time, when bubbling stops and the liquid goes from green to dark brown/black, then add some new H2O2 (20%). Continue, until all H2O2 is
added. Then wait, until really no more copper is dissolved. This takes some time.
Now, pour the solution in a large excess of water. An amazingly large amount of very white CuCl is precipitated in the form of small crystals. The
precipitate is not slimy, it is a compact crystalline precipitate, which quickly settles. I tried to isolate this precipitate, but as soon as some of
this is exposed to air, it discolors, it becomes green/brown. I did not succeed in obtaining a nice dry, still white sample.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by woelen
@blogfast25: The CuCl-precipitate is obtained if you dissolve a lot of copper metal (as much as possible) in a mix of equal volumes of HCl (30%) and
H2O2(20%). Add a lot of copper wire (the thin wires from electricity cord is best) to conc. HCl and then slowly with stirring add H2O2 (20%) in small
steps. Each time, when bubbling stops and the liquid goes from green to dark brown/black, then add some new H2O2 (20%). Continue, until all H2O2 is
added. Then wait, until really no more copper is dissolved. This takes some time.
Now, pour the solution in a large excess of water. An amazingly large amount of very white CuCl is precipitated in the form of small crystals. The
precipitate is not slimy, it is a compact crystalline precipitate, which quickly settles. I tried to isolate this precipitate, but as soon as some of
this is exposed to air, it discolors, it becomes green/brown. I did not succeed in obtaining a nice dry, still white sample. |
Yep, I'm definitely trying that. My H2O2 is less concentrated (I believe, haven't looked for a while) but it should work nonetheless.
I have seen CuCl drop out of Cu(NO3)2 solutions reduced with Cu or metasulfite many times before but I then end up with a dark green solution which
seems to resist hydrolysis very well and is quite stable.
Have you tried filtering the CuCl quickly on a Buchner? Washing carefully with freshly boiled (but cooled, of course) water? My experience is that,
yes, it does oxidise easily but perhaps not as quickly as you describe.
According Wiki, it's prepared industrially by direct chlorination of copper metal. There's a rainy day project, I guess...
The three solutions (1:4 diluted complex, further diluted complex and the mix of 1:4 and control), all yielded precipitates with varying hues of
green. Again to me this suggests not a great deal of hydrolysis has taken place upon dilution, because then I'd expect some clean blue from Cu(OH)2.
From very concentrated solutions of the complex things may of course be very different.
My three test tubes are now in place:
#1: a few mL of 0.1 M CuCl2, plus aq. dist + 0.4 g of copper wire - cyan colour
#2: same amount of 0.1 M CuCl2, saturated with NaCl + 0.4 g copper wire - green colour
#3: same amount of 0.1 M CuCl2 in 32 w% HCl + 0.4 g of copper wire - green colour, slightly darker than #2.
If I don't get any CuCl, I might still run the molar Cu(2+)/Cu(+) experiment with 0.1 M CuCl2.
I'm taking some pics too...
|
|
|
| Pages:
1
2
3 |