| Pages:
1
2 |
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Recovering a sulphated lead acid battery
I replaced the lead acid battery on a car for a friend and got the old battery. The car had not been used for more than six months and the old battery
would not charge up. When I tried ot charge it with my intelligent (so called) charger the charge was not able to even detect that it was connected to
a battery.
Out of curiosity I decided to attempt to charge the battery from a high voltage source, a half wave rectified 230Vrms via a 25W 240V bulb. The bulb
light up perhaps a little dimmer than I would have expected.
After a few hours I checked the battery voltage and it was 14.2V. About 24 hours later the battery voltage had dropped to 12.6V and if I shorted ut
the battery I get a slight spark initially but the wire did not heat up. I left it charging for a further day and then tried it on the intelligent
battery charger. It now detected the battery but reported the battery was almost fully charged with a voltage of 13.9 volts.
I left the battery charging for 3 days. The voltage gradual dropped to abut 12V and the charger indicated the charged was decreasing. Then gradual the
voltage increased to 13.5 and the charger indicated it was getting charged. The charger now reports the battery as fully charged at about 13.7V and if
I short out the battery there are large splashed as I connected the shorting wire which gets hot very quickly.
\I don’t know how much charge the battery can hold or if it could crank an engine some additional experiments will be need for that. I assume the
battery was sulphated and my high voltage charge has decomposed at least some of the sulphation.
Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Interesting. I hadn't expected any recovery of the energy storage function, but you proved otherwise! I had one as well and took it apart, hoping to
salvage the lead in there. (A very long-term project I'm still working on.) I found short cuts in some of the cells (membrane had a hole and the
electrodes were touching), so I guess it wasn't worth much anymore.
Please let us know if you have any indication of how much of the storage capacity has been recovered.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
SLR batteries use paper thin plates, a great many of them, on any thing at or below 50% discharge they suffer physical damage due to crystal growth on
the positive plates. all so active material will flake off (this is why they have the plastic socks around them) As this happens you lose capacity
Sulphation is reversible to a degree and depending on how valuable the battery is. drain and wash the batteries cells, refill with distilled water
then push the voltage to what ever is needed to get a steady 4amps.
Keep close watch on amps and temp, as time goes on the Sg of the water will rise as the hard sulphate goes into solution, if the Sg gets close to
original electrolyte dump and add fresh water and keep going until no change is noticed (This is arbitrary it is up to you to decide how desperate you
need the battery thus time and effort invested)
Take the original electrolyte, filter and boil till clean, dilute to the Sp stated by battery manufacturer.
To play with batteries in any useful way you need: Temp corrected Sg(Sp) meter, DC Current meter, good voltage meter, load bank (Car head light will
do) and a current/voltage adjustable charger
[Edited on 23-10-2018 by XeonTheMGPony]
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bezaleel  | Interesting. I hadn't expected any recovery of the energy storage function, but you proved otherwise! I had one as well and took it apart, hoping to
salvage the lead in there. (A very long-term project I'm still working on.) I found short cuts in some of the cells (membrane had a hole and the
electrodes were touching), so I guess it wasn't worth much anymore.
Please let us know if you have any indication of how much of the storage capacity has been recovered. |
I put a 20W, 1.67A (the only bulb I had handy) load on the fully charged (according to the charger) battery. The battery is specified as 75A.h with a
cold cranking current of 600A. No significant drop in voltage after one hour so it has a capacity of at least 1.67A.h. With that load the battery
voltage dropped 50mV that’s an internal resistance 0.03ohm. The battery is supposed to be able to supply a cold cranking current of 600A at a
minimum of 7.2V indicating a battery resistance no greater than 0.008ohm. That’s does not look good. Ok I don’t expect the resistance to be
constant over that current range but I would guess the resistance goes up at high currents not down.
If the bulb remains on for 24 hours that indicates 40A.h abut half the rated capacity which is apparently about correct for an old car battery and
will probably be ok for battery back up lighting.
At some point I will probably make a high current load to test the cranking current or at least try cranking an engine.
Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by XeonTheMGPony  |
Sulphation is reversible to a degree and depending on how valuable the battery is. drain and wash the batteries cells, refill with distilled water
then push the voltage to what ever is needed to get a steady 4amps.
|
Xeon: Do you know why the acid must be replaced with water in that desulphation process or what is the theory behind the process?
What does SLR stand for if its not a camera?
[Edited on 24-10-2018 by wg48]
Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
SLR = Starting, lights running battery.
Hard sulfate is far more soluble in pure water then in acid mix, it pushes things to being more faverable for removing it back to solution.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bezaleel  | Interesting. I hadn't expected any recovery of the energy storage function, but you proved otherwise! I had one as well and took it apart, hoping to
salvage the lead in there. (A very long-term project I'm still working on.) I found short cuts in some of the cells (membrane had a hole and the
electrodes were touching), so I guess it wasn't worth much anymore.
Please let us know if you have any indication of how much of the storage capacity has been recovered. |
With a discharge current 1.67A for 25.2 hours the voltage has dropped to 11.8, indicating a capacity of 42A.h. As I will be away for a few days I must
terminate the discharge experiment.
Below is plot of the voltage v hours. The top point at zero time is the OC voltage before the discharge was started.
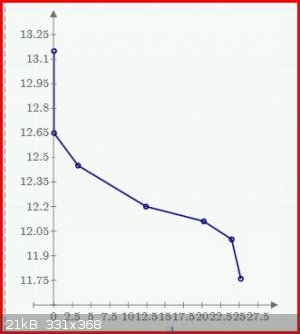
Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
standard test is 5 amps for 20 hours for determining ampacity.
8h rate will be higher AH when tested on a 20h rate
https://batteryuniversity.com/learn/article/bu_503_how_to_ca...
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
I was given a 6V gell type lead acid battery that was believed t be dead by the original owner. So I thought I would try the high voltage source,
(half wave rectified 230Vrms via a 25W 240V bulb) method of de-sulphating the battery.
Initial the battery had a terminal voltage of less than one volt. When first connected to the high voltage source the terminal voltage was 15V on a
digital multimeter. Considering the half wave source the 15V corresponds to a peak voltage of more than 30V when current is flowing. The 15V dropped
about 0.1V per second so after a few minutes the voltage was 5.9V and about the same with the high voltage source turned off.
I will leave it charging then try a capacity test on it.
Having had success with two previous batteries one of which was a gell type its starting to look like this is a method to recover some sulphated
batteries.
Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
The 6V battery has now been charging at approximately 100mA for 12 hours. Its voltage has risen from 5.9V to 6.6V and can now supply approximately 8A
to a 55W 6V bulb for at least several seconds.
Apparently what I assume was a discharged and sulphated battery had a resistance of at least 3,000ohm. Apparently high voltage charging drives
sufficient current through the battery to reduce its resistance (now less than 0.1ohm) and charge it slowly.
Probably the charger used by the original owner did not have a sufficiently high charging voltage to de-sulphate the battery or it took too long to do
so or if it was a "smart" charger it did not recognise a battery was connected.
I also have an old car battery that has not been used for at least three years so I assume its sulphated. I will attempt to recover it.
Note that I know that car batteries are frequently replaced not because they are sulphated (not accepting a charge) but because their capacity is
reduced or their cranking current is too low such that they no longer reliably start the car in adverse conditions.
Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I, and several colleagues, have tried various lead-acid battery recovery techniques over the decades,
it is often not too difficult to resurect a battery, d.c., pulsed d.c., charge/discharge cycling .....
the zombie batteries are OK for non-critical use,
but it will not be long before a zombie battery fails.
Unless desperately poor, I would not use a zombie battery in my car for example,
the cost and inconvenience of a non-cranking battery outweighs any short term extension of battery life.
Consider how much experience and expertise has been involved with lead-acid cells over the last 150 years,
and the potential profit in selling a resurection method .......
yet all commercial attempts at battery resurection kits have faded away.
In my 'lab' Sealed/VRLA/gell batteries such as the Yuasa range aimed at standby operation rarely last more than three years, sometimes five.
I have a Yuasa NPC24-12 battery that I have had in intermittent use for over ten years,
I do not know the internal difference between normal, deep-cycle etc. but for me the extra cost has been worth it.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
sodium_stearate
Hazard to Others
  
Posts: 255
Registered: 22-4-2011
Location: guard duty at the checkpoint
Member Is Offline
Mood: No mask.
|
|
The zombie batteries are good for small loads
such as a tail-light lamp.
Last summer I resurrected a hopelessly sulphated
and discharged 12 volt lead acid battery that had been
sittiing around dead for over one year.
Hooked it up to a plain simple old 1 amp. trickle charger
for a few days. After adding some distilled water and
a couple day's charging, it holds enough of a charge to
light up a small license plate lamp for about an hour.
That is good enough for what I needed.
That is for illuminating a railroad switch lamp
in a rail museum's yard, at night, for a photo-shoot.
PS....I got no help from any of the other lazy volunteers
at that outfit, for assistance in carrying that damn heavy
worn-out old truck battery way out into the rail yard to
set it up.
But yeah, if all you need is a short interval at
very low current, old ruined lead-acid batteries can
be coaxed into actually holding a very marginal charge.
"Opportunity is missed by most people
because it is dressed in overalls and it
looks like work" T.A. Edison
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
De-sulphating a lead acid battery that had other problems prior to the sulphating is not likely to have those other problems fixed or more accurately
I have no evidence that it does.
The two batteries I had already de-sulphated had measured capacities more than 50% of the capacity printed on their labels. The car battery was from a
vehicle that was only used for holiday excursions. I don't know the history of the gel battery it looked newish.
The gel battery I am presently de-sulphating was from a rechargeable torch (flash light). It reached its standby charging voltage with less that 30%
of its rated charge input. I suspect it will have limited capacity when I measure it.
Apparently sulphation of a lead acid battery can increases the internal resistance of the battery to such an extent that a normal trickle charge with
an open circuit voltage of only a few volts more than battery voltage can not charge it and "smart" chargers may not even attempt to do so as they
cannot determine that a battery has been connected or determine if its a 6V or 12V battery.
Yes de-sulphating a battery does not necessarily fix its broken plates, shorted plates, low capacity plates or replace lost electrolyte.
Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
I did a crude capacity test on the torch battery (see one of my previous posts) by reconnecting it to the torch and leaving it switched on. The light
went out some time between 17min and 20min after it was turned on. The bulb is rated at 55W which will draw about 9A. So assuming 20min that’s 3A.h
almost 50% of the 6.5A.h stated on the battery. That’s good for an old battery.
So I decided to try with an other gel lead acid battery that has been sitting an a shelf in my workshop for about two years. It’s a 12v 3A.h from a
battery strimmer.
When I connected up to the half wave rectified 240V via the 25W 240V bulb, the bulb glowed a deep red and flicked. The voltage across the battery was
93V as measured by my DMM which is probably averaging the rectified voltage so the peak voltage will be about 186V. The not charging battery voltage
was 6V.
After about 20min the flickering stopped and the on charge battery voltage has dropped to 85V. After 15 hours the on charge voltage had dropped to
12.1V with not charging voltage of 11.6V. I hooked the battery up to a 50W 12V bulb and to my surprise it light up brightly. Eventually I will do a
capacity test on it.
So far I have recovered/de-sulphated one car battery and three small gel cells. The first three had measured capacities of about 50% or more (car
battery). Of cause I don’t know how long they will remain reasonable good for and I may have been lucky with the batteries that had not been abused
a apart from them having been left discharged. Judging from my small sample this de-sulphating method works very effectively. As previously stated
it only de-sulphates the battery. If the battery has other problems prior to its sulphating it will probably still have them after it is de-sulphated.
Note that most battery chargers can not supply a charging voltage of 186V so they would take several days to de-sulphate or never. Apparently some
“smart chargers have a special mode to charge what they describe as a fully discharged battery. I suspect the mode is a high voltage low current
trickle charge similar to my method.
Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
I have been using the the torch that contains the 6V gel type lead acid battery that was desulphated.
At the moment the battery seems to be near to being discharge as the light dimes after several seconds., but I have noticed an odd effect. It now dims
briefly (less than a second) and then recovers to almost the same brightness as when it was first switched on. I have never noticed that before with
any lead acid battery. Perhaps its the gel or an effect from the sulphation and desulphation.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
https://en.wikipedia.org/wiki/Peukert%27s_law
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
That does not account for bright then dim then almost as bright effect I spoke of. ie not a simple exponential drop in output volts.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
It is another effect I am trying to recall, but that's a start.
Crack of the whip, it has to do with crystal growth and seed crystal formations
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by wg48temp9  | I have been using the the torch that contains the 6V gel type lead acid battery that was desulphated.
At the moment the battery seems to be near to being discharge as the light dimes after several seconds., but I have noticed an odd effect. It now dims
briefly (less than a second) and then recovers to almost the same brightness as when it was first switched on. I have never noticed that before with
any lead acid battery. Perhaps its the gel or an effect from the sulphation and desulphation. |
i found a dead car lead acid battery that did the same. it would run a motor fine for 1 second and then drop the voltage until the motor stopped,
disconnecting the motor and reconnecting it after a few seconds made this cycle again and again but with a shorter power curve. at the end the battery
was totally flat
maybe i'll try your method to recover it
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by Ubya  | Quote: Originally posted by wg48temp9  | I have been using the the torch that contains the 6V gel type lead acid battery that was desulphated.
At the moment the battery seems to be near to being discharge as the light dimes after several seconds., but I have noticed an odd effect. It now dims
briefly (less than a second) and then recovers to almost the same brightness as when it was first switched on. I have never noticed that before with
any lead acid battery. Perhaps its the gel or an effect from the sulphation and desulphation. |
i found a dead car lead acid battery that did the same. it would run a motor fine for 1 second and then drop the voltage until the motor stopped,
disconnecting the motor and reconnecting it after a few seconds made this cycle again and again but with a shorter power curve. at the end the battery
was totally flat
maybe i'll try your method to recover it |
It does not sound like your battery is sulphated. A sulphated battery is high impedance at least hundreds of ohms with a an open circuit voltage as
low as 1V. It would not be able to operate the starter solenoid much less turn the engine. I suggest you try to charge it with a regular charger
first.
In addition if the battery is not high impedance the circuit I used will do nothing more than charge it slowly.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I just use a bench power supply to recover lead-acid batteries,
- set to 30 Vdc with current limiting
Initially negligible current is drawn from the psu but after hours or days the battery will draw current at the current-limit setting of your psu,
then reduce the charging voltage to suit your battery.
Be 'gentle' when charging a recovered battery, C/10 or preferably less.
I've done this many times, most lead-acid batteries 'recover' to some extent or other, but it has been long-term successful on only a few occasions.
_____
(my 'best' source of usable rechargeable cells has been laptop Li 18650 battery packs,
almost always the electronics failed -not the cells, very occasionally one or more bad cells)
(with a 4-cell battery holder you get a convenient replacement for 12 V battery power)
(not so long ago there was a good chance that your made-in-China cheap rechargeable 'widget' was/is internally powered by an ex-laptop cell 
-----
P.S. I consider higher voltages to be;
a) hazardous
b) un-necessary - just faster for the impatient.
12V battery = 6x 2V cells, 30V = 5 V/cell which is more than enough for any likely electrochemistry - I believe
[Edited on 24-2-2019 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
roXefeller
Hazard to Others
  
Posts: 463
Registered: 9-9-2013
Location: 13 Colonies
Member Is Offline
Mood: 220 221 whatever it takes
|
|
Quote: Originally posted by wg48temp9  |
It does not sound like your battery is sulphated. A sulphated battery is high impedance at least hundreds of ohms with a an open circuit voltage as
low as 1V. It would not be able to operate the starter solenoid much less turn the engine. I suggest you try to charge it with a regular charger
first.
In addition if the battery is not high impedance the circuit I used will do nothing more than charge it slowly. |
Is full sulphation then the expected status of a battery that is deeply discharged? I've read here, post #8, that batteries discharged well below 1.75 V per cell are unrecoverable, at least to normal capacity in the opinion of the
manufacturers. He later mentions, #14, that the charger won't help the tested battery and only heat is formed. Given that the SG of the electrolyte
is near 1.0, the sulphate group should be all over the plate instead of the water. Obviously the charger isn't set up for Xeon's prescription, nor
yours.
I ask because in my electronics hobby I use rechargeables often as portables. I take smaller AGM types for air travel but I also use larger AGM and
flooded depending on my Ah needs. While I was reconditioning one flooded battery, I noticed that another, which had been on a solar
charger/maintainer, had the solar leads chewed in half by a groundhog or something. The load for that battery goes to DC regulators and a bus of
devices, so it has likely been drained substantially over the last year. Likely down to the low voltage limit of those regulator transistors. I
haven't had a chance yet to pull the battery for depth of discharge but the open circuit voltage may be into the 5V or lower range. It may be a good
chance for an additional experiment.
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by roXefeller  | Quote: Originally posted by wg48temp9  |
It does not sound like your battery is sulphated. A sulphated battery is high impedance at least hundreds of ohms with a an open circuit voltage as
low as 1V. It would not be able to operate the starter solenoid much less turn the engine. I suggest you try to charge it with a regular charger
first.
In addition if the battery is not high impedance the circuit I used will do nothing more than charge it slowly. |
Is full sulphation then the expected status of a battery that is deeply discharged? I've read here, post #8, that batteries discharged well below 1.75 V per cell are unrecoverable, at least to normal capacity in the opinion of the
manufacturers. He later mentions, #14, that the charger won't help the tested battery and only heat is formed. Given that the SG of the electrolyte
is near 1.0, the sulphate group should be all over the plate instead of the water. Obviously the charger isn't set up for Xeon's prescription, nor
yours.
I ask because in my electronics hobby I use rechargeables often as portables. I take smaller AGM types for air travel but I also use larger AGM and
flooded depending on my Ah needs. While I was reconditioning one flooded battery, I noticed that another, which had been on a solar
charger/maintainer, had the solar leads chewed in half by a groundhog or something. The load for that battery goes to DC regulators and a bus of
devices, so it has likely been drained substantially over the last year. Likely down to the low voltage limit of those regulator transistors. I
haven't had a chance yet to pull the battery for depth of discharge but the open circuit voltage may be into the 5V or lower range. It may be a good
chance for an additional experiment. |
Yes a sulphated battery is a fully discharged battery but a fully discharged battery that has to be left in that condition for many days for the
sulphation to form. From my experience many days is anything from a week to months.
I assume you are referring to the poster who fully discharged a battery (from memory down to 5V open circuit or on load was not specifically stated).
He then attempted to recharge the battery and discovered although current did flow into the battery (the battery got hot) it did not hold a charge.
Assuming he was charging the battery with a ordinary charger that has an open circuit voltage have about 15V than the battery was not sulphated as a
sulphated battery would allows negligible current to flow from 15V.
One of the batteries I desulphated was a gel type battery in a torch and since its "reanimation" I used it. While it was left in my car the battery
became discharge to the point that on switching on no brief glow or any redness of the filament could be seen. I would expect that to mean that
battery voltage on load (the bulb) was less than half the the normal voltage. Several days later I successfully charged it with an ordinary charger
and have used it since though not for sufficient time to to be confident that it has the capacity it previously had.
I should also add that the battery care note of one lead acid battery manufacture recommended immediately recharging a fully discharged battery
preferably within one day or the battery could be damaged.
Is your battery you use lead acid ? You can buy capacity tester I think I already mentioned it. I have not used mine yet.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
roXefeller
Hazard to Others
  
Posts: 463
Registered: 9-9-2013
Location: 13 Colonies
Member Is Offline
Mood: 220 221 whatever it takes
|
|
My lead acid battery has definitely gone beyond several months. I checked it yesterday and found it at 2.74 V The current tapers off quickly on a
smart charger, starting around 4A to 1 or 2 and a quickly rising voltage. I should take a peek at the plates. Anyway, I have a manual bulk charger
that should provide the necessary transformer, rectifier, and fuse for the experiment. I'll redo the primary windings to provide a variable tap,
reminiscent of a tapped variac. My lab current supply doesn't go above 1 amp.
The link I gave has two people. The OP claims there's no problem going down to 5V and back. A responding post provided practical experience about
draining to 7V and inability to charge afterward, with only heat ensuing.
|
|
|
roXefeller
Hazard to Others
  
Posts: 463
Registered: 9-9-2013
Location: 13 Colonies
Member Is Offline
Mood: 220 221 whatever it takes
|
|
Here are pictures from the set up I'm running today. I've drained the battery out it's roughly a 60 amp hour battery. I replaced the electrolyte with
distilled water. The first picture is of the DC power supply. It is a old bulk charging battery charger from 10 or 12 years ago. I repurposed it to
have variable taps on the primary and maintain use of the full wave rectifier. The second photo is the meter I'm currently running around 6 amps and
14.5 volts. It's been chewing now for an hour.
  
|
|
|
| Pages:
1
2 |