MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
Looking for a TCT source
I accidently posted this on a thread that nobody wants to read.
I have been calling around to some chemical distributors in my area to no avail. Only one place so far even knew what I was talking about and they
only sell to businesses. I can't find any internet sources. I was wondering if anyone had any advice on what kind of places to call and what to tell
them I am using it for because I'm sure if I told them I was using it for experimentation they would hang up on me. I am traveling to New Orleans in
a few weeks and will be there for a few months. Does anyone know of any good distributors in the NOLA/LA or Little Rock AR area?
Thanks in advance....
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Your problem is calling it TCT. Try cyanuric chloride, or 'stabilized pool chlorinating agent'.
Comes in big jars for use in swimming pools and hottubs.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
I thought TCT = Cyanuric chloride (2,4,6-Trichloro-1,3,5-Triazine)
TCCA = Trichloroisocyanuric acid (1,3,5-trichloro-1,3,5-
triazinane-2,4,6-trione)
and TCClA is used for pools.
Correct me if I'm wrong.
I read that NaCN? can be oxidised with Cl2 and then polymerized to TCT but the intermediate is extremely toxic and polymerizes explosively.
[Edited on 15-11-2007 by MagicJigPipe]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Cyanuric chloride is correct alternative nomenclature. "Stabilized pool cleaner" is an invitation to confusion between CC aka TCT, and TCCA. TCCA is
used as pool cleaner, TCT as far as I know is NOT.
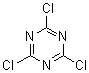
CC or TCT is trichloro-s-triazine.
[108-77-0]
2,4,6-Trichloro-1,3,5-triazine; 2,4,6-Trichloro-s-triazine; 2,4,6-Trichloro-1,3,5-triazine
Molecular Formula: C3Cl3N3
The chlorines are on carbons.
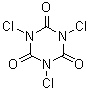
TCCA is N,N,N-trichloroisocyanuric acid. The chlorines are on nitrogen.
1,3,5-Trichloro-1-triazine-2,4,6(1H,3H,5H)-trione; Symclosene
Molecular Formula: C3Cl3N3O3
CAS [87-90-1]
Two very similar but chemically very different molecules. TCCA is useful but not for chlorinating carboxylic acids.
When in dount, always specify by the CAS registry number.
The structure below is CC or TCT.
The acid chloride of cyanuric acid. The cyclic trimer (-N-C(Cl)-)3 in other words, cyanogen chloride.
While TCCA is the chloro-amine of isocyanuric acid.
MJP gets full marks. The Dayster, rather uncharacteristically, gets to sit in the corner and wear a pointy hat.
By the way the principle uses of CC/TCT are in the manufacture of agro-chemicals, i.e., weed killers and pesticides. See Ullman's chapter on cyanuric
acid and derivatives.
[Edited on 16-11-2007 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I believe someone on this board mentioned buying it as a pool chem, although I myself have looked and only found the TCCA.
You do have me doubting myself now, I may have just confused them in my head.
EDIT Hmm yes, I do believe I made a mistake, apologies.
[Edited on 15-11-2007 by The_Davster]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Never mind, old friend. It is a common mistake, and the fault is really the over-reliance on acronyms.
So, what is your hat size?
Structure below is TCCA. You can imagine it as the cyclic trimer ((-C=O)-N(Cl)-)3
[Edited on 16-11-2007 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
Well, what do you know? Another case of Acronym confusion. Nothing like a graphic formula for cyclics! Lest I sound like a broken record, please never
use ******* acronyms without defining them at least once...
Regards, Der Alte.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The plain cyanuric acid dihydrate is what is used as a chlorine stabilizer in swimming pools .
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
get the CAS number and you'll eliminate the confusion
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The confusion about these two is nothing new, it happened on The Hive, it has happened on this forum a few times.
Both are cyclic triazines. Neither contain hydrogen.
But the one contains 3 oxygens, and the other none.
That, and watch for the Cl connectivity.
The CAS numbers make it safe, and the correct nomenclature is very unambiguous.
Always good advice from my pal O3.
Sic gorgeamus a los subjectatus nunc.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Surprisingly other individuals who must know better have had trouble differentiating these two
http://www.sciencemadness.org/talk/viewthread.php?tid=4094&a...
Lets put this to rest _
https://sciencemadness.org/talk/viewthread.php?tid=2726&...
You'd be surprised what you can find on Wikipedia despite what detractors say
CYANURIC CHLORIDE ( 2,4,6-trichloro-1,3,5-triazine )
http://chemicalland21.com/specialtychem/perchem/CYANURIC%20C...
The precursor for Cyanuric Triazide by way of NaN3
 . . . Note - Nitrogen in the top ' 1 ' position . . . Note - Nitrogen in the top ' 1 ' position
CYANURIC ACID
http://chemicalland21.com/industrialchem/organic/CYANURIC%20...
From
Kirk-Othmer Encyclopedia of Chemical Technology _
Nomenclature is based on the keto–enol tautomers. The trihydroxy form is variously
designated cyanuric acid, s-triazine-2,4,6-triol or 2,4,6-trihydroxy-s-triazine. The
trioxo structure, or s-triazine-2,4,6(1H,3H,5H)-trione is the basis for the isocyanuric
acid nomenclature. The triazine-based system is preferred in the scientific literature
and is used by government regulatory agencies; however, the less cumbersome
(iso)cyanurate designations persist in commerce. The keto form normally predominates
in cases of keto-enol tautomerism. In alkaline solution, the anion formed is that of the
hydroxy tautomer. Through common usage, both forms are collectively called
cyanuric acid (CA).
easily homemade
https://sciencemadness.org/talk/viewthread.php?tid=8160&...
useful for producing Cyanamide , so I have read here _
http://www.sciencemadness.org/talk/viewthread.php?tid=8594&a...

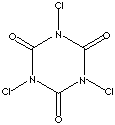
TRICHLORO-ISOCYANURIC ACID ( 1,3,5-trichloro-1,3,5-triazine-2,4,6-trione )
N-Chlorination of Cyanuric acid produces chloroisocyanurates that are widely used as
disinfectants, sanitizers, and bleaches. TCCA is prepared by chlorination of Cyanuric
acid with chlorine gas. Increasingly popular as a mild chlorinating agent , TCCA has
a low 1.2 % solubility in water. However, it is very soluble in Acetone and organic
solvents. Cyanuric acid is insoluble in Acetone.
http://www.chemicalland21.com/industrialchem/organic/TRICHLO...
.
[Edited on 30-11-2007 by franklyn]
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I think I definitely would not like to swim in a pool with TCT in it. I value my life too much  . .
I also have been looking around for this one (Sauron has raised my interest in this), but I cannot find any source for this. It is a rather nasty
chemical, so I guess it will be difficult to obtain.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
It's not that nasty. Merck describes its odor as "pungent" but, it's a solid and has a whole lot less odor than a lot of rather routine liquid
reagents I can think of.
A foreign student at a US university last year got into a fender-bender and become upset when the driver of the other vehicle called the police. The
student had some TCT in his car, supposition being that he pilfered it from school but who knows? Anyway he opened the bottle and began rubbing the
TCT all over his own face.
I don't know why, or what he thought would happen, but basically nothing did happen. Did he think the TCT would east his skin? Render him
unrecognizable? Kill him? Anyway he was detailed for psychiatric observation and to assist the police (and maybe federal authorities with their
enquiries. He was some sort of middle east type (need I mention).
Apart from some superficial irritation the TCT had no effect whatsoever on his skin. The level of irritation was consistent with friction (like
rubbing sand into your face) rather than chemical attack.
Bizarre.
Compared to other chlorinating agents, like conc HCl, SOCl2, PCl3, PCl5, oxalyl chloride, benzoyl chloride, phthaloyl chloride etc. - TCT is the only
one I would care to work with outside of a hood, on an open benchtop. Whether or not I would do so would depend on the nature of the product rather
than on the behavior of the TCT.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Phosphor-ing
Hazard to Others
  
Posts: 246
Registered: 31-5-2006
Location: Deep South, USA
Member Is Offline
Mood: Inquisitive
|
|
I have seen "trichloro-s-triazine" on a pool chemical label at walmart before. Of course it is not there now as it is too cold but, I'll keep an eye
out for it next time I'm near pool/spa supplies.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
trichloro-s-triazine? Are you sure? Although, I suppose it's not entirely impossible because of it's reaction with water. And about the acronym
thing, I thought that this was discussed enough that it has become a well known acronym. Sorry, if there was any confusion.
When I first heard of 2,4,6-Trichloro-1,2,3-triazine I too thought, "holy shit, that's the pool chemical stuff" but when I did a quick google search
on it I realized it wasn't.
According to these people TCT reacts with water to form cyanuric acid and HCl. I suppose it could be useful as a pH lowerer. If it were actually a
pool chemical.
http://www.dfmg.com.tw/member/chemical/cas/108-77-0.htm
[Edited on 17-11-2007 by MagicJigPipe]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
TCT does react with wayer to form cyanuric acid and HCl. The reaction is irreversible.
TCCA reacts with water to form cyanuric acid and Cl2.
Cyanuric acid reacts with Cl2 gas to form TCCA. But chlorinating cyanuric acid to TCT is very difficult. It requires expensive reagents and so is
uneconomical (very) compared to its industrial preparation. One such reagent is PCl5 which thanks to DHS is now in the unobtainium class. The other is
oxalyl chloride, which requires a deep pocket and a fume hood and protective equipment/apparel. The reaction produces a lot of CO. Obviously, 3 mols
oxalyl chloride are required per mol TCT and in practice - more. This is an expensive way to make TCT.
Replicating that preparation on a bench scale is EXTREMELY dangerous.
Sic gorgeamus a los subjectatus nunc.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
I didn't want to start a new post on this so...
Would a condenser made of copper be acceptable for most alcohols/alkanes/ethers? I ask because I just rigged one up for MeOH/EtOH for speed purposes
and if it's acceptable for other things then.... great.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Not really. Alcohols oxidize to acids, acids react with copper and you end up with blue/green products.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Nitro-esteban
Harmless

Posts: 39
Registered: 10-4-2013
Location: Fifth dimension
Member Is Offline
Mood: inert
|
|
Does trichlorotriazine react with water? If it does not, perhaps it could be made by reacting trichloro triazinetrione with hydrogen peroxide.
C3Cl3N3O3 + 3H2O2 = 3O2 + C3N3Cl3 + 3H2O.
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The reaction you describe here certainly does not occur. If you add H2O2, then the H2O2 will act as reductor, being oxidized to oxygen and the TCCA is
converted to cyanuric acid and the chlorine ends up as HCl (which ionizes to make the liquid acidic). Secondary reactions will occur between the HCl
and TCCA in which Cl2 and cyanuric acid are formed.
TCT cannot be formed from TCCA, at least not through any easy route. TCT is a completely different beast, despite the similarity of name and
similarity of formula.
|
|
|