| Pages:
1
2 |
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Fresh Look at Sulfur Halide Rxn w/Acyl Salts
A page in the Rhodium archive refers to a Chemistry & Industry article in the 40s taken from the Russian literature, detailing a lab prep of acid
anhydrides from anhydrous alkili and alkiline-earth salts of carboxylic acids. These are reacted with sulfur chloride (S2Cl2) or sulfur bromide
(S2Br2) either preformed, or prepared in situ. The reaction is fast and the yields high. Obviously the sulfur halide serves to convert part of the
salt into acid chloride which then reacts with more acid salt to form the anhydride.
However, looking over the procedures it is clear that the reaction is more complex, at least in some of the examples. Nowhere is complete
stoichiometry given, and in the very first example, a large excess of bromine is employed relative to both sulfur and sodium acetate.
So what exactly is going on here?
22 g sulfur (0.65 gram-atom)
320 g Br2 (4 gram-atoms or 2 mols of diatomic bromine)
440 g NaOAc anhydrous. 5.5 mols This is described as fused at 320 C. I believe this is an error, as NaOAc loses all its water at 125 C and starts to
decompose above that.
The sulfur is dissolved in the bromine first, and the NaOAc added last with stirring.
Obviously less than two thirds of a gr-atom of S will only consume the same amount of Br2 to form a little less than 1/3 of a mol of S2Br2 or about 75
g worth. This reacts with the same quantity of NaOAc to form AcBr and NaBr. The byproduct is SO2 and sulfur according to the equation
2 S2Br2 + 2NaOAc -> 2AcBr + 2NaBr + SO2 + 3 S
AcBr + NaOAc -> Ac2O + NaBr completes the overall reaction.
That balanced equation appears nowhere in the Rhodium page (attached). I do not know whether or not it appears in the original Russian publication or
in the Chem&Ind translation which I have not seen. I worked it out for myself.
The sulfur will of course form more S2Br2 as long as bromine remains. Whether or not SO2 reacts with bromine under these conditions I do not know.
Normally a catalyst is required to form sulfuryl halides (e.g., GAC or camphor.) So we will assume that sulfuryl bromide does not form.
Another procedure describes the use of preformed S2Cl2, and in this case SO2 is vigorously liberated, which was not noted in the above procedure with
sulfur and excess bromine.
I believe this adds to the understanding of this reaction. It would be interesting to try the reaction of SO2Cl2 with anhydrous NaOAc to see if this
works. Perhaps sulfuryl bromide can form without a catalyst, I will check and see. But even if so it is a minor participatent in this reaction.
PS Mellor indicates that SO2Br2 is unlikely to exist, at least above liquid SO2 temperatures, so I think we can discount any such involvement.
Propionic Anhydride
40 g anhydrous sodium propionate 0.42 mol
2 g sulfur 0.063 gr-atom
22 g Bromine 0.3 gr-atom
Yield 25 g
Those numbers track well with the acetic anhydride prep, except that the bromine is less. (shrug) To get a direct comparison multiply by 11
440 g sodium propionate
22 g sulfur
242 g bromine
So one gr-atom bromine is missing and the excess of salt has been reduced, the FW of sodium propionate is 96 and that of sodium acetate 82. The ratio
of salt to bromine is about the same in both cases.
--------------------
In another example, NaOAc is reacted with a stoichiometric amount of S2Cl2 preformed reagent and then distilled to give Ac2O, NaCl, 3 S and SO2. The
authors however propose that the reaction procceds as:
2 NaOAc + S2Cl2 -> AcO-S-S-OAc + 2 NaCl
2 AcO-S-S-OAc -> 2 Ac2O + 3 S + SO2
No reference is given so I can't tell is the original investigators supported their proposed mechanism in any way. I will have to look and see is
diacetyl disulfide has been isolated and characterized. Anyway, even if not it ought to be possible to halt this procedure before distilling
(pyrolyzing) the supposed sulfide and isolating it, if it exists.
In which case this reaction may not involve an acyl halide at all. Wouldn't that be interesting? It would imply that this diacyl disulfide might be
stable enough to store and allow preparation of Ac2O or other acid anhydrides as needed. How interesting.
PS Apparently diacetyl disulfide does indeed exist. It turns up as an impurity in commercial thioacetic acid, and can be regarded as the dimer of
that. (AcS)2 from 2 AcSH I am still hunting for its preparation. Thioacetic acid, if I recall, is nasty stinky toxic stuff,
PPS The general method of converting thiols to disulfides is to use I2 as a catalyst. So thiolacetic acid, prepared per Org.Syn. by treating acetic
acid with H2S, is reacted with I2 giving diacetyl disulfide. If the Russian authors being quoted in Rhodium are correct, this substance on
distillation will be quantitatively converted to acetic anhydride.
Thiolacetic acid is a toxic material of very persistent stench. Thus far I have not found a commercial source for the diacetyl disulfide. All in all,
this sounds like a great way to turn one's lab into a real skunk works. If the reaction of sulfur, bromine and sodium acetate (anhydr.) was not noted
to be particularly stinky, then I suspect that diacetyl disulfide may not have been produced - unless it is unlike thiolacetic acid in its nasty
smelling nature.
[Edited on 6-9-2007 by Sauron]
Attachment: Ac2O.pdf (34kB)
This file has been downloaded 1448 times
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
As mentioned, there's an Org.Syn. procedure for preparing thioacetic acid (thiolacetic acid) Ac-SH from H2S and acetic anhydride. The great August
Kekule, however, prepared this from glacial acetic acid and P2S5 (P4O10) in 1854 as described in Ann., 90, p 309. I will post this shortly as it may
be more convenient, and it is less circular than procedures requiring Ac2O or AcCl.
Also I do not have a cylinder of H2S, but I do have phosphorus pentasulfide!
Thioacetic acid is available from Aldrich (expensively) or Acros (half the Aldrich price.)
A procedure for oxidation of thioacetic acid to diacetyl disulfide is described in a US patent (attached below) as Example XI. The oxidant is DMSO and
catalyst is conc HCL with a trace of I2 or HI. Yield 75% Diacetyl disulfide is a low melting solid mp 8-15 C which may well explain the odd
description of the reaction mass in the Rhodium article. ("Semisolid semiliquid")
I am not seriously proposing thioacetic acid and diacetyl disulfide as a prep route to Ac2O. But it would be very interesting to prepare diacetyl
disulphide by the patent route and then distill it to see if it pyrolyzes to Ac2O, S and SO2.
My feeling is that the best variation on this reaction to prepare Ac2O is to use preformed S2Cl2 which is available as byproduct from chlorination of
CS2, on the way to making CCl4. This makes use of a byproduct, rather than consuming large quantites of bromine in the making of S2Br2 in situ.
But others may have different priorities.
Kekule's 1854 paper proposes preparation of thioacetic acid by distilling it from phosphorus pentasulfide.
5 AcOH + P2S5 -> 5 AcSH + P2O5
300 g glacial acetic acid
222 g P2S5
380 g thioacetic acid (theoretical yield)
142 g P2O5
In practice I'd use excess AcOH and reflux till the yellow pentasulfide has been converted to white pentoxide.
The bp of thioacetic acid is 93 C so efficient fractionation will be necessary to seperate it from the excess AcOH.
So far I have not noticed any yield stated by Kekule.
[Edited on 7-9-2007 by Sauron]
Attachment: 3954800.pdf (367kB)
This file has been downloaded 1034 times
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
And here's the Kekule paper from Ann., vol 30 p 309. (1854)
It turns out P2S5 is not as expensive as I recalled, I must have been cheated on the Merck product.
Acros sells a Kg of phosphorus pentasulfide for less than $50.and this is enough to convert acetic acid to almost 2 L of thioacetic acid so maybe 1.5
Kg diacetyl disulfide and therefore 1 Kg or more of Ac2O.
So this is competitive with other methods of preparing Ac2O and cheaper than some.
[Edited on 7-9-2007 by Sauron]
Attachment: kekule.pdf (309kB)
This file has been downloaded 1091 times
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
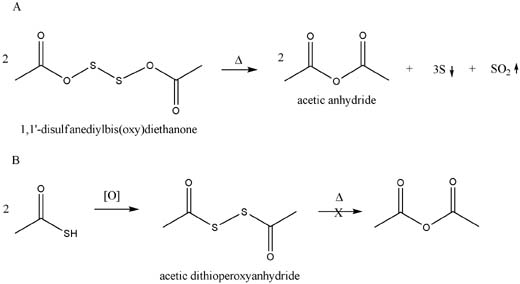
Although the (Rhodium) equations are not balenced, e.g. 2 moles of the 1,1'-disulfanediylbis(oxy)diethanone will yield 2 moles of acetic anhydride,
three of S and 1 of SO2. Thermodynamically this look like it will work.
Pondering:
But, in order for this to occur, we would need to see a concerted reaction involving 2 or more equivalents of the diethanone (or the molecule would
probably fall appart whilst liberating SO2).
done pondering.
Unless I am missing something, though, the bisdithioketone from thiolacetic acid is not equivalent and should not yield acetic anhydride via the same
mechanism. Perhaps this might work with a large excess of air or some hydrogen peroxide (and heat)--might get frisky!
Please let me know if I missed something (patents are a pain when your in a hurry).
Cheers,
O3
[edit] Although my German is terrible, this, from Kekule, looks interesting and on the right track:
"Reaction zeigt , erhalt man wohl ein intermediares Anhydrid
von der Formel :
C2H3O-S-OC2H3"
On the attached figure I don't like the "peroxyanhydride" bit that the Chemoffice naming algorithm coughed up. I meant to remove it, but forgot. oops.
[Edited on 7-9-2007 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Well done, I overlooked that difference.
Thanks for the correction.
SO I will look for an alt prep of (CH3C(O)OS)2.
C4H6O4S2
I just drew that in MDL ISIS Draw 2.5 w/AutoNom and the latter barfed on naming this, gave an undefined structure error message. I ran Clean molecule
and still get same error.
So do you think this compound unlikely as intermediate? Unlikely to exist?
If so then my original hypothesis (halogenation by S2X2 of NaOAc to acetyl halide followed by attack on more NaOAc) is more likely.
Kekule indeed reported that acetic anhydride treated with P2S5 gives acetyl sulfide MeC(O)OSOC(O)Me
If this compound pyrolizes to Ac2O (extruding sulfur ) then indeed I'd say this is a possible intermediate. But whence comes the oxygen?
---------------
If S2Cl2 (or S2Br2) form (AcOS)2 as reported by the Russian investigators and Rhodium, then it follows that SCl2 (there is no SBr2) ought to give
(AcO)2S.
We are taught (by the Russians) that the reaction of S2X2 with NaOAc gives upon pyrolisis, Ac2O. And we are taught by Kekule that we can make the
(AcO)2S from acetic anhydride and P2S 5. Can it too be pyrolized with or without P2O5?
[Edited on 8-9-2007 by Sauron]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I wanted to follow-up on this thread with some related information and a question. Also at Rhodium and next to the process cited above is this one:
Propionic anhydride. To 40 g. fused and powdered sodium propionate in a flask of 250 cc. capacity a solution of 2 g. sulphur in 22 g. bromine was
added while stirring. The temperature was kept at about 50 C. When the operation was completed, the anhydride was distilled off in vacuo. The crude
product (25g.) was fractioned under normal pressure, and the fraction 155-156 C was collected. Yield, 23 g. propionic anhdride of 90% purity = 85%..."
This looks a lot more attractive but there's a point (con)fusing me. Why is the acid salt "fused?" Is it assumed it was just made from the acid and
thus could have water in it? Could there be another reason? If one buys reagent Na-prop could he proceed without the fusion?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
sodium propionate is deliquescent, it is best to make sure your salt is anhydrous before the reaction. The same goes for trying to make say, acetic
anhydride from sodium acetate and acetyl chloride, any water present will really kill your yields so it is best to fuse the salt to minimize loss of
product due to water.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I was evidently mislead with a consequent waste of material and lessons learned. The TCT method makes a-chloro-acids not acid chlorides.
Solo placed a pdf in refs. I copied it below.
[Edited on 1-2-2008 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemrox
The TCT method makes a-chloro-acids not acid chlorides. |
No it does not. That would make for a redox nonsense. Count the electrons.
BTW, your link points to no post relevant to cyanuric chloride. Please correct it or provide the PDF.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
a-Cl-acids via TCT paper
@Nicodem- a usually reliable source may want to re-think
PS_ I would feel relieved to learn I had misread this or reinterpreted
CRX
[Edited on 1-2-2008 by chemrox]
Attachment: a-chlorination acids using TCT.pdf (304kB)
This file has been downloaded 1999 times
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
a-Chlorination of Carboxylic Acids using Trichloroisocyanuric Acid (TCCA) is not using Cyanuric Chloride (TCT)
Déjà Vu!... Another case of abbreviations causing mayhem...
[Edited on 1-2-2008 by PainKilla]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
oh s**t
Thanks Painkilla, apologies to Nicodem and others. I'm still confused by this reagent and will shortly report an experiment using it to make acid Cl.
Caught a flu that caused a hiatus and may explain some of the confusion.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
failed anhydride experiments
Experiment 1 was a scale up from the sulfer/bromine method and it failed in the sense that most of the acid Na salt remained unreacted. Two possible
factors; inadequate drying-the salt sat a long time after fusing and powdering, the other factor, inadequate stirrring. I found it impossible to move
the stuff inisde the flask sufficiently to get good mixing.
The other failure was also a scale-up with some of the same issues. The method was Na salt plus acyl halide. The addition of the halide did not
produce a lot of heat right away. When the addition was complete it looked like twice as much halide could have been added. Distillation of the
resultant mix started at 65 degC and ended at ~160 degC
acyl halide bp 80; acid bp 141 and anhydride bp is 168 go figure. Azeotropes? There was a lot of leftover material to recover after all was done.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
I am very surprised by this method. It is essentially identical to the time-honoured preparation except all in one pot and with one exception, there
is no SO3. The normal chain is
S + Cl2 -> SCl2 + SO3 -> SOCl2 + AcOH -> AcCl + AcONa -> Ac2O.
Here all the ingredients are mixed in one pot (normally this lowers yield) minus the SO3. So if this works SOCl2 overkill and S2Cl2 should do in the
above sequence. Somehow I doubt it. Ac2O, AcOCl are all highly hydroscopic. As a rule synthesis of such reagents in good yield requires a
dehydrating step or a dehydrating agent, such being SOCl2 and SO3.
[Edited on 10-4-2008 by len1]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
refer me to the one you're citing if you don't mind. I still need a supply of about 300 ml acid anhydride and I'm getting tired of spending precious
lab time this way. Thanks! PS I edited because I'm very confused by what you're telling us and thinking I miscommunicated. As I understand it,
SOCl2 is one way of making the acid Cl. I have the acid Cl from another method. So my "one-pot" is reacting the acid Cl with the Na salt as you
indicated. I don't see how you break that one down further. Please clarify. Or have I done?
[Edited on 9-4-2008 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Im sorry, I think there has been a misunderstanding. I was referring to the method at the top of this page, with Ac2O from S, Br2, and NaOAc
directly. If you have the AcCl from another source this does not apply to you. As far as Im aware reacting this with anhydrous NaOAc + AcCl->Ac2O
+ NaCl should work as ive seen that reaction documented in many places (but have never tried it myself).
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Len1:
That reaction you cited may look fine on paper but in practice things are different
While you can at risk of life and limb employ SO3 to prepare SOCl2 there is a better safer way that has been described on this site more than once.
Phthaloyl chloride is easily prepared from phthalic anhydride and benzotrichloride per procedure of Thomas Kyrides, byproduct is benzoyl chloride,
also very useful.
Phthaloyl chloride reacts with SO2 (dry) and catalytic amound ZNCl2 to give SOCl2.
HOWEVER
AcOH and SOCl2 does NOT produce a practical yield of AcCl. This has ALSO been stated many times on this forum, and can be found in Vogel's chapter on
acid chlorides. The bp of SOCl2 is too close to that of acetyl and propionyl chlorides to allow facile separation by fractionation. This results in a
highly impure product. Vogel recommends using thionyl chloride for C4 acids and higher.
The practicable methods for preparing acetyl chloride (or propionyl chloride which is chemrox's target) are benzoyl chloride (method of H.C.Brown,
posted here many times); phthaloyl chloride (method of Kyrides, posted here many times); TCT in acetone with Et3N and extracting the acid chloride
from pptd cyanuric acid with DCM; or use of oxalyl chloride (method of Roger Adams, posted here many times) which can also directly prepared the
anhydride from the acid.
The two methods of those four most likely to produce best results are benzoyl chloride and TCT, the phthaloyl and oxalyl chlorides being relatively
expensive..
All of the methods involving anhydrous sodium acetate or propionate are best done NOT by dehydrating sodium acetate trihydrate (which has to be done
twice) but by purchasing the best grade anhydrous NaOAc available such as Merck super-dry product, and using it directly from freshly opened bottle.
Gattermann recommends fusing even such anhydrous product, once. But everyone I know of who has tried this gets a lot of charring. Apparently it is
rather technique intensive and scale sensitive. IMO, seek other methods.
[Edited on 10-4-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by len1
I am very surprised by this method. It is essentially identical to the time-honoured preparation except all in one pot and with one exception, there
is no SO3. The normal chain is
S + Cl2 -> SCl2 + SO3 -> SOCl2 + AcOH -> AcCl + AcONa -> Ac2O. |
I don't understand what makes you think it is similar (surely it can not be identical) to what you described?
The reaction discussed in this thread is about the thermal decomposition of the very unstable AcOSSOAc (I guess one can call it "diacetyl
sulfoxylate") and related intermediates to acetanhydride.
Of course the reaction can only give yields above 0% if the sodium acetate contains less than 10% water which is not easy to achieve. But then again
that is the problem with all routes to acetanhydride starting with sodium acetate anyway.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
The similarity is in the starting materials and the product - the reactions suggested could well be different, but the start and end point is the
same. What is surprising is that the essence is a huge shortcut in the chain I described and in the absence of a strong dehydrating agent. Ac2O
generally requires a strongly dehydrating environment in order to form - I was expressing my doubts that the reaction presented represents such an
environment, its another way of looking at things. I could be wrong, the test is in the pudding.
@Sauron I was using SOCl2 as a reagent capable of forming acid chlorides, of this there is no doubt. Separability is not at issue, rather reactivity
is. As a practical method for AcCl SOCl2 has the disadvantage you mention. Kyride's reaction, which I see you have been a big fan of is interesting
in its own right though.
[Edited on 10-4-2008 by len1]
[Edited on 10-4-2008 by len1]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I'd buy the Merck super-dry grade NaOAc, in a packing size that I could use all at once, and see what sort of yield I could get right out of the
bottle, no fusion. If yield is depressed, I'd do a single fusion with next batch and see if charring can be avoided and what the yield is. I have a
forced-air, controlled temperature drying oven by Memert, that ought to do it. I believe it heats to 250 C. The required temperature is not that high.
(It is stated incorrectly in Rhodium). NaOAc starts to decompose >125 C.
Sure, the anhydrous salt costs more than the trihydrate but your savings are ephemeral when you are paying for 3H20/mol and then charring a lot when
trying to drive that off.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by len1
The similarity is in the starting materials and the product - the reactions suggested could well be different, but the start and end point is the
same. What is surprising is that the essence is a huge shortcut in the chain I described and in the absence of a strong dehydrating agent. Ac2O
generally requires a strongly dehydrating environment in order to form - I was expressing my doubts that the reaction presented represents such an
environment, its another way of looking at things. I could be wrong, the test is in the pudding. |
But this particular reaction involves no dehydration. Actually all the reactions (that I can currently think of) leading to either acid chlorides or
acid anhydrides involve either a rearrangement of heteroatomic functions (for example: RCOOH + SOCl2 => RCOCl + SO2 + HCl and all similar ones) or
acylation of a carboxylate (for example, RCOO<sup>-</sup> + RCOCl => (RCO)2O + Cl<sup>-</sup> . .
If carboxylic anhydrides could be prepared by dehydration, then plain old H2SO4 would suffice since it would give a thermodynamically allowed
equilibrium, yet it appears it does not work even though H2SO4 is more than enough strong acid to form the RCO<sup>+</sup> required for
the acylation of RCOOH.
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
This is going along the lines of our previous argument about decarboxilation to pyridine. You are arguing about reaction mechanisms, I say one has
little confidence is theorising about these with certainty - careful and tedious experiments have to be done. Thermodynamics is something with
simpler and more certain results. Two things about thermodynamics:
1) It only needs the start and end point - doesnt give two hoots about reaction mechanisms - hence my comment about the similarity of the processes -
2) Thermodynamics can only predict if a process is possible not if it will occur - and thats where your comment about H2SO4 falls in - it is
thermodynamcally allowed but does not occur. Thats standard.
Heres what thermodynamics says.
The starting and end points are:
AcOH -> Ac2O + H2O delH = a
so whaever the steps, this is a dehydration of AcOH, because water is released on the RHS.
a is positive so the reaction wont occur by itself, we need a dehydrating agent to drive the equilibrium. Here are the differences I was referring
to:
1) SOCl2 + H2O -> SO2 + 2HCl delH = b for the standard process
2) S2Cl2 + H2O -> 1/2SO2 + 2HCl + 3/2S delH = c
It does not matter whether these are or are not the actual reactions occuring at the molecular level. They correspond to the start and end products.
Thermodynamically that is full stop.
Formation enthalpy SOCl2 ~ -246 kJ/mol
Formation enthalpy SO2 ~ -298 kJ/mol
Formation enthalpy S2Cl2 ~ -60 kJ/mol
So delH for second reaction is 30kJ/mol more negative. This means S2Cl2 is actually a stronger dehydrating agent than thionyl chloride, contrary to
what I was expecting. So yes there is no thermodynamic reason why reaction cant occur. Doesnt mean it will actually take place though. I find it
strange though that people use SOCl2 in the dehydrations if S2Cl2 will do.
To be fair we should actually use delG rather than delH. That is however quite dependent on reaction conditions. Nonetheless because 1) releases
1/2mole more of gas this narrows the gap between the two processes.
[Edited on 11-4-2008 by len1]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
As the thread author and someone who knows something about the subject of acyl chlorides and anhydrides I would just like to reiterate that thionyl
chloride has nothing whatsoever to do with the subject of this thread.
Thionyl chloride is one of a number of reagents for making acyl chlorides. That is taught is every taxtbook or organic chemistry.
The authors of those texts are often uninterested in the fine print and exceptions. THOSE teach us that SOCl2 is unsuitable for aliphatic acid
chloride preps lower than C4.
Both acetyl chloride and propionyl chloride are < C4.
Furthermore, Nicodem is perfectly correct. While for pedagogical purposes the first year texts present formation of acid anhydrides as union of two
mols of R-COOH into 1 mol of RCO-O-OCR with abstraction of 1 mol H2O - that is simply neither how it happens nor how it is done.
Here are examples of carboxylic anhydride formation:
NaOAc + AcCl -> Ac2O + NaCl.
Where's the water? There was no water in the reactants and there's no water in the products.
To find any of the elements of water we's have to go back to when the AcOH was turned into a salt and the other AcOH was turned into an acyl halide.
The first produced NaOAc and H2O. The second, probably HCl.
Two seperate reactions both offstage.
Similarly
2
NaOAc + S2Cl2 - AcOSSOAc + 2 NaCl
No water. None on either side of equation.
Pyrolyze that and you get Ac2O and still no water.
How about reagents that proceed to Ac2O from AcOH in one pot, one step?
Like Oxalyl chloride.
Roger Adams teaches us in JACS 90 years ago that AcCl is formed first and then this reacts with AcOH, products are HCl and (from the oxalyl chloride)
CO and CO2.
Even in the cases of dicarboxylic acids of the right chain length to form cyclic 5 and 6 membered anhydride rings, the mechanism is not a simple
abstraction of water.
As Nicodem correctly pointed out, were it otherwise you could prepare anhydrides by use of powerful dehydrating agents like H2SO4 or P2O5 (P3O10).
This however does not work.
The mechanism is if not everything - almost everything.
[Edited on 11-4-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
  
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Correction
Actually, in the special case of some dicarboxylic acids, e.g., those with C4 or C5 chain lengths such as succinic and maleic acids, phthalic acid,
etc. it is possible to prepare the anhydride by direct abstraction of water, such as by heating the dicarboxylic acid in an inert high boiling solvent
(Vogel suggests tetrachloroethane).
The formation of such cyclic anhydrides by reaction of the open chain dicarboxylic acid with acetic anhydride can also be regarded as a dehydration
reaction.
But with acetyl chloride, although the reaction still proceeds to an anhydride and AcOH, this is not formally a dehydration.
Anyway this is merely a special exception, these procedures do not apply to intermolecular anhydride formations, only intramolecular ones. I just
wanted to be accurate and fair in my remaarks.
[Edited on 11-4-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
| Pages:
1
2 |