MEXCHEM2006
Hazard to Self
 
Posts: 50
Registered: 22-8-2006
Location: MEXICO
Member Is Offline
Mood: No Mood
|
|
Methylation by reduction
I need to prepare N-methylamino acids by reduction of their N-benzyloxycarbonyl derivatives , but i dont have lithium aluminum hydride at hand , i
was thinking in using NaBH4 but i dont have information of this type of reduction with this or other reagents , maybe someone has some experience in
this reaction and can help me .
Thanks in advance.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I think you will have to remove the protecting group first, Then you can methylate the amino group.
You might avail yourself of the two peptide books some saintly benefactor put up on 4shared.com
Principles of Peptide Synthesis
The Practice of Peptide Synthesis
Both 2nd Edition.
http://www.4shared.com/dir/2245331/5a78115f/sharing.html
Benzyloxycarbonyl is usually abbreviated Z (like Zorro) in honor of Emil Zervas, the Max Bergmann coworker who developed this reagent and PG. In the
old literature it is referred to as carboxybenzyl, and the Chinese still stubbornly abbreviate it as Cbz. But just Z is more common.
The classical removal of the Z group is by catalytic hydrogenation at atmospheric pressure over Pd/C in toluene. This was also developed by Bergmann
and Zervas. Since the PG falls apart to toluene and CO2 this affords a very clean product and unusually easy workup.
[Edited on 30-8-2007 by Sauron]
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
A General One-Pot, Three-Component Mono N-Alkylation of Amines and Amine Derivatives in Lithium Perchlorate/Diethyl Ether Solution
Mono-N-Alkylation of Amines
Akbar Heydari,* Hossein Tavakol, Saied Aslanzadeh, Jamshid Azarnia, Nafiseh Ahmadi
Synthesis 2005: 627-633
Abstract:
An efficient, general procedure for reductive monoalkylation
of amines and amine derivatives with aldehydes is reported.
Treatment of aldehydes with primary amines, secondary amines, Otrimethylsilylhydroxylamine, and N,N-dimethylhydrazine in lithium
perchlorate/diethyl ether and trimethylsilyl chloride, followed
by BH3·NEt3 reduction, and straightforward workup afforded secondary
amines, tertiary amines, N-substituted hydroxylamines, and
hydrazines, respectively. Reductive alkylation of a-amino esters
with aldehydes afforded the corresponding N-monoalkylated aamino
esters selectively.
Key words lithium perchlorate, reductive amination, alkylation,
amines
Attachment: A General One-Pot, Three-Component Mono N-Alkylation of Amines and Amine Derivatives in Lithium Perchlorate iethyl Eth (1.3MB) iethyl Eth (1.3MB)
This file has been downloaded 2423 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
MEXCHEM2006
Hazard to Self
 
Posts: 50
Registered: 22-8-2006
Location: MEXICO
Member Is Offline
Mood: No Mood
|
|
Thank you both for the information , but what i want to do is to react the amine with benzyl carbamate , and then reduce it with other reagent than
lithium aluminum hydride , when you reduce the N-benzyloxycarbonyl derivative with lithium aluminum hydride it automatically removes the protecting
group and methylates the amine .
References
J.Heterocyclic Chem 13 , 775 (1976)
Synthetic communications 28(11) 1935 (1998)
Helv.Chim.Acta 35 , 1581 (1952)
J.Org.Chem 1562(1975) H.E.Smith
Vogel pg.762
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
Reduktionen einiger einfacher Peptide mit Lithiumaluminiumh y &id
von P. Karrer und B. J. R. Nicolaus.
Helv.Chim.Acta 35 , 1581 (1952)
Attachment: Reduktionen einiger einfacher Peptide mit Lithiumaluminiumh y &id.pdf (370kB)
This file has been downloaded 715 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
Synthesis of S(+) and R(-& #8203 - & #8203; 3- &
#8203;(2-Aminopropyl)indole from Ethyl- & #8203; D- and L- & #8203;Tryptophanate - & #8203; 3- &
#8203;(2-Aminopropyl)indole from Ethyl- & #8203; D- and L- & #8203;Tryptophanate
David B. Repke , Wilfred J. Ferguson
J.Heterocyclic Chem 13 , 775 (1976)
Abstract
A stereospecific requirement for hallucinogenesis applies to certain molecules containing an asymmetric center. Thus, only the R- isomers of
substituted phenylisopropylamines and lysergic acid diethylamide are psychotomimetic. The enantiomers of a minor hallucinogen, S(+)- and
R(- -& #8203;3-& #8203;(2-aminopropyl)indole
(a-methyltryptamine) (6a and 6b) were synthesized via a 5-step manipulation from D- and L-tryptophan ethyl ester
hydrochloride, respectively. Optical purity of these two isomers was determined by pmr spectroscopy of their complexes with a europium chiral shift
reagent using the indole C2H signal. -& #8203;3-& #8203;(2-aminopropyl)indole
(a-methyltryptamine) (6a and 6b) were synthesized via a 5-step manipulation from D- and L-tryptophan ethyl ester
hydrochloride, respectively. Optical purity of these two isomers was determined by pmr spectroscopy of their complexes with a europium chiral shift
reagent using the indole C2H signal.
[Edited on 1-9-2007 by solo]
Attachment: Synthesis of S(+) and R(- -3-(2-Aminopropyl)indole from Ethyl-D- and L- (238kB) -3-(2-Aminopropyl)indole from Ethyl-D- and L- (238kB)
This file has been downloaded 902 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
A SIMPLE AND EFFICIENT PROCEDURE FOR
THE SYNTHESIS OF OPTICALLY ACTIVE 4-METHOXYa-
METHYLPHENYLETHYLAMINE FROM TY ROSINE
Harumichi Kohno, Takeo Iwakuma,') and Koichiro Yamada
Synthetic communications 28(11) 1935 (1998)
Abstract:
Optically active 4-methoxy-a-methylphenylethylamin(e l ),a useful chiral building block in medicinalchemistry. was synthesized from L- orDtyrosine by
using a simple andefficient prccedure via one-pot zinc reduction of the corresponding 0-tosylate (4) in the presence of H,O and Nal in pure form
Attachment: A Simple and Efficient Procedure for the Synthesis of Optically Active 4-Methoxy-agr-Methylphenylethylamine from Tyrosin (519kB)
This file has been downloaded 1097 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Optically Active Amines. XIX.' Circular Dichroism of Ortho-, Meta-, and
Para-Substituted &Phenylalkylamine Hydrochlorides. Further
Applications of the Salicylidenimino Chirality Rule2
Howard E. Smith,*3a Elizabeth P. and Fu-Ming Chen[/i
J. Org. Chem., Vol. 40, No. 11, 1562,1975
Abstract
0- and m-chloroamphetamine have been resolved and the absolute configurations of the respective enantiomers determined by catalytic hydrogenolysis to
amphetamine. The established absolute configurations of (R J-(-)-o-, (S)-(+)-m-,a nd (S)-(+)-p-chloroamphetaminea re compared with predictions based
on Snatzke's sector rule for the correlation of absolute configuration with the sign of the ILb Cotton effect and with predictions based on the
salicylidenimino chirality rule for the correlation of absolute configuration with the sign of the Cotton effects near 255 and 315 (325) nm in the CD
spectrum of the N-salicylidene (N-5-bromosalicylidene) derivative. Snatzke's rule does not correctly predict the sign of the lLb Cotton effect for the
meta isomer. The salicylidenimino chirality rule, however, correctly predicts the sign of the Cotton effects near 255 and 325 nm observed for the
N-5-bromosalicylidene derivatives, the S configuration in each case producing strong positive Cotton effects. These results confirm the
configurational assignments for the nonstimulant anorectic agent (+) -fenfluramhe and for the enantiomers of the psychotomimetic amine
l-(2,5-dimethoxy-4-methylbenzyl)ethylaminme,a de on the basis of the
CD spectra of the respective N-salicylidene derivatives.
Attachment: Optically active amines. XIX. Circular dichroism of ortho-, meta-, and para-substituted .beta.-phenylalkylamine hydrochl (722kB)
This file has been downloaded 872 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
But none of those references seem to contain a one step amine methylation and deprotection by reduction of it's N-benzyloxycarbonyl derivative with a
reducing agent other than LiAlH4?
(Interesting chemistry though  ) )
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
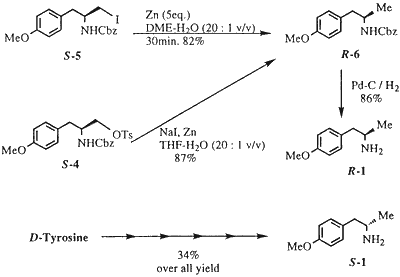
Here benzyloxycarbonyl is used to protect the amine , but upon removal via catalytic hydrogenation using Pd 10% no methyl group remained only the
primary amine......
Optically Active 4-Methoxyamphetamine from Tyrosine
Harumichi Kohno, Takeo Iwakuma and Koichiro Yamada
Synthetic Communications 28(11), 1935–1945 (1998)
source......,
http://www.erowid.org/archive/rhodium/chemistry/tyrosine2pma...
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|