woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
chemiluminiscence
This is an experiment I did with plain swimming pool chemicals (TCCA and Na-DCCA). Very simple to do, but the effect is quite stunning. Some of you
may know this already, but probably many others of you don't. This is something I want to share with you:
http://woelen.homescience.net/science/chem/exps/chemlum/inde...
EDIT(woelen): Changed link, so that it works again.
[Edited on 20-1-20 by woelen]
|
|
|
ssdd
Hazard to Others
  
Posts: 211
Registered: 13-4-2007
Location: Central Canada
Member Is Offline
Mood: Hypergolic
|
|
This is quite ironic that I planned to run this same experiment this week. 
But the instructions I found were a bit different, I'll post the instructions for anyone interested.
Pour several ml of 30% H2O2 into a test tube. Add a small amount of Sodium Hydroxide to this. If the Hydrogen Peroxide begins to break down to fast
add water until it slows down. Slowly bubble chlorine gas through this mix and you will get the red glow.
From reading your page I would say your method is safer and simpler for doing this.
My only question is, I lack in 30% peroxide... would 3% work at all, even for a weak result. (I'd be adding it to Calcium Hypochlorate.)
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
I think the reaction would be extremely fast and weak due to the dilution. I just tried with 15% and I had a fraction of a second of bright red as the
mix frothed, filling a 200ml flask completely, then nothing. I think this is because the DCCA dissolves completely very fast in the larger volume of
liquid unlike with the 30% It sounds very much like the reaction described with calcium hypochlorite.
EDIT: Quick Question, is the Na-DCCA you used hydrated, because mine is listed as such which may be the cause of my problem.
[Edited on 5-28-07 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
When I get home, if I remember, I'll try to get a video of luminol chemiluminescence. Gives a really bright blue, very cool.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
| Quote: | | EDIT: Quick Question, is the Na-DCCA you used hydrated, because mine is listed as such which may be the cause of my problem. |
I used simple swimming pool chemicals, without a precise specification. The bottle is labeled as: sodium dichloroisocyanurate, at least 60% active
chlorine.
I do not, however, think that the use of an hydrated version of this salt affects the outcome of this experiment very much. What does matter, however,
is the concentration of the H2O2 and how finely the Na-DCCA is divided.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
The Na-DCCA was prilled (55% active chlorine, 99% min purity) The H2O2 was 15% (maybe slightly less due to its age).
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
ssdd
Hazard to Others
  
Posts: 211
Registered: 13-4-2007
Location: Central Canada
Member Is Offline
Mood: Hypergolic
|
|
I ran it this morning with 3% and TCCA in a flask. I only got a fairly vigourous bubbling and frothing of the liquid. (About 60ml of H2O2 and a gram
of TCCA.) There was some dim light in the room and perhaps this is why I saw nothing... Perhaps I'll put out a few dollars and buy the 30%. 
________
What if I added a small amount of Sodium Hydroxide to this? From what I read it needs to be activated by something...
Any thoughts?
[Edited on 29-5-2007 by ssdd]
|
|
|
phj
Harmless

Posts: 18
Registered: 7-5-2007
Location: Netherlands
Member Is Offline
Mood: inquisitive
|
|
Isn't it possible to perform the blue luminol reaction and the singlet oxygen reaction in one system, hence creating a purple glow?
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I don't think so. The luminol reaction requires hydrogen peroxide as oxidizer. I think you will have a very hard time, finding a set of chemicals,
which are compatible with each other and allow the reactions to run independently.
Another problem is the time scale. The luminol reaction is much longer lasting, so if you could manage to obtain the red glow at the same time, then
it still would only be for a short time, relative to the time of the "glowing" luminol.
|
|
|
Per
Hazard to Others
  
Posts: 134
Registered: 26-1-2007
Location: Europe
Member Is Offline
Mood: No Mood
|
|
I tried this attemp today with Ca(OCl)2 and 30%H2O2 and it worked great, red light flashes appeared after dropping the peroxide.
Best results werte obtained by adding just only one drop at the same time and then wait a little bit, when the reaction mix bubbles to much you can´t
see anything of the light.
luminol:
It´s possible to increase the intensity the intensity of the blue light very hiht by adding sodium hypochlorite to the shining luminol reaction mix,
that gives very vigorous flashes of blue light and I only tried that with a 2.4% solution.
Also the addition of KMnO4 cristals is interesting because at the places where the cristals falls into the reaction mix it shines a bit yellowish,
just like a starry sky.
|
|
|
Armistice19
Hazard to Self
 
Posts: 87
Registered: 19-6-2007
Member Is Offline
Mood: Brain sponge activated!
|
|
Now I could be wrong here, but I really don't think I am. I'm fairly certain that if you take 3% Hydrogen Peroxide and boil it in a beaker the result
is a decomposition reaction that releases oxygen, and leaves water as the final product, But if you gently heat the H2O2, and bring it to a suttle
steaming temperature without boiling it, it should concentrate the H2O2 into 30% 40% or 50% depending on the length of time that it is heated.
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Na DCCA is the best. NaOCl reacts too fast, TCCA too slow. DCCA speed is accurate.
This experiment is very attractive, not only to us, but also especially for children.
I made a short video Na DCCA + 30% H2O2
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Oh that is cool, didn't know that is also an option.
I have a whole liter of, formerly containing 30% H2O2, which is a few years old and certainly not of much use anymore.
Maybe that will still be sufficient for a chemoluminescence reaction?
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
karlos³ I used 30% H2O2 which was certainly older than 10 years but apparently it was stabilized so its concentration was still very high (other
decade old plastic flasks with unstabilized formerly 30% H2O2 contained only H2O without any H2O2).
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Thank you for that tip, I had a look and it also says stabilised on the bottle.
Although a second bottle, likewise old, performed the times I used the last bits up somewhat worse than at the times I bought it.
But that information gives me confidence in that the stuff is not totally degraded and I can still use it well 
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
I determined the H2O2 concentration, only approximately:
1,00 ml of H2O2 released 130 ml of O2 at 15 C (temperature of water used for capturing the gas and the reaction vessel was at the end also submersed
into water with the same temp and allowed to equalize temp as decomposition of H2O2 is exothermic and warms the gas, so I let it to cool down to
defined temperature not only in capturing measuring calibrated cylinder but also in the reactor vessel - 100 ml reactor vessel containing 1 ml H2O +
0,1 g MnO2 prior slowly injecting H2O2 - and only after equalizing temperatures (little of gas sucked back into reactor) I removed the hose from the
graduated cylinder.
Wow the concentration is still slightly more than 30% although label says 30% - I expected it to be below 30% as most of producers wants to earn as
much money as possible (while I had a summer job in vinegar factory the bottles of vinegar claimed 8% concentration but the norm allowed it to be in
range 7,84 - 8,16% and it was always diluted to the 7,84 %...).
The volume of H2O2 injected into reactor was measured with error less than 2% and the gas volume with error less than 1%.
I have 2 bottles of this 30% H2O2 and both yielded 130 ml of O2 from 1 ml samples. They are certainly older than 15 years. It was a gift to my brother
(much more bottles and not only H2O2) - we produced O2 which we captured into plastic bottles from beverages, we left few ml of H2O in every bottle,
then went outside into isolated places, there we dropped a piece of CaC2 into bottle, closed the bottle with a cap with blaster wire and ignited
electrically... I'm sure how old they are. My brother does not need them anymore, they stayed in the home where our parents are living.
Volumetric determination of the H2O2 concentration was much faster than preparing solution of KMnO4, standardizing permanganate using oxalic acid and
finally titration of H2O2. Also less wastes produced and less risk of spilling and coloring something with permanganate. 20 years ago I would titrate
it with KMnO4.
I remember that I also bought few kg of KMnO4 for my brother and no KMnO4 left in my parents home, my bro used it all into flash powder KMnO4+S+Al :-D
|
|
|
Dr.Bob
International Hazard
    
Posts: 2750
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
You might be able to use a dry to increase the light output, as singlet oxygen can react with some dyes to transfer the excited state, I believe.
Other good chemoluminescent demo is luminol dissolved in DMSO with solid NaOH in a sealed container. Blow some fresh aitr into a sealed containe of
it, and shake well, the oxygen can react directly (but slowly) in this solvent system to oxidize the luminol and form a small amount of light. The
good part is that as it is slow, the solution will last a long time before decaying too much to work.
Also, tetrakis(dimethylamino)ethylene will glow green when exposed to oxygen or air. This was tested by the Air Force as an agent for spotting
planes downed in water, but was found to be to impractical, as not cheap and no way to shut off the light if the enemy approaches once released. It
floats on water, so lots of surface area to react. This makes it ideal for puting about 1 ml into a toilet, which will then glow a scasry shade of
green. This might have been done during a party at a dorm in the RA's bathroom, where some poor girl looked in and saw the glowing toilet, screamed,
"Holy shit", and another partier looked in and said "I think it is!", which caused everyone to laugh histerically. The bad news is that the chemical
smells of dimethylamine, which smells like rotting fish, even in the smallest amounts.
Lastly, making the oxalate diesters is always fun, did that one a few times, and then TA'd 45 kids making it in gradual school many years ago. A few
good good yields, and some of them managed to do the oxidation carefully enough to make a pretty bright light. But many others made a big mess, and
little if any light, so a good test of organic skills. This oxidation requires fairly pure H2O2 dissolved in a thick organic solvent, which is not
safe or easy to make at home, but can be done in theory. You also must have a dye here, as the excite state glows in the UV unless coupled to a
lower wavelength dye. But a great experiment. See cyalume lightsticks on wiki for details.
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by Dr.Bob  |
Also, tetrakis(dimethylamino)ethylene will glow green when exposed to oxygen or air. This was tested by the Air Force as an agent for spotting
planes downed in water, but was found to be to impractical, as not cheap
|
Unfortunately that tetrakis(dimethylamino)ethylene compound apparently is very difficult to make requiring some exotic, hard to buy and expensive
reagent. It has been briefly discussed here http://www.sciencemadness.org/talk/viewthread.php?tid=8037#p...
Below is its very symmetrical structure and the oxidation reaction.
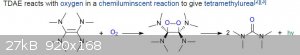
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|