Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Isolating cobalt, copper and lithium from an old LiPo RC Helicopter battery.
I created a solution of chemical salts from an old RC helicopter battery. I only used one cell out of three and dissolved the contents using HCl,
H2SO4 and a bit of H2O2 diluted to 15%. The solution sat for 24 hours, then I heated it to a boil to further dissolve as much as I could from the
battery contents and to decompose any remaining H2O2.
I got a dark red solution after vacuum filtration, then proceeded to add aluminum foil until dissolution came to a crawl. I vacuum filtered the solid
contents and placed the solution aside for further extraction later on.
The solids I collected from solution contain a lot of copper contamination from the battery. I'm pretty sure I obtained most of the lithium during a
previous unmentioned phase using oxalic acid to form lithium oxalate--small white needle shaped crystals. I also got some lithium carbonate
precipitation while concentrating the solution through heated evaporation, but moving onto my main focus of separating the cobalt from the copper.
I decided a possible way I could isolate each element was to strongly heat the solids in carbon. As I understand it, both cobalt and copper
precipitated in elemental form when displaced from solution during the previous reaction using aluminum. I figure, copper oxide is reduced to
elemental copper using carbon in a redox reaction, so my hypothesis was that copper will remain in elemental form and melt together into a bead, while
the cobalt should form cobalt carbide. I'm still researching and experimenting to see if this is truly the case.
Once I observed no additional molten material forming in the reaction, I continued to heat for about another five minutes. I allowed the contents to
cool then pour out the remains onto a piece of aluminum foil. I'm thinking if this is a mixture of Cu and CoC, then I could add the material into a
beaker of H2SO4 and allow the CoC to dissolve in the acid, while the copper should for the most part be untouched and remain as a solid in
solution.--I've already completed all stages except for dissolving the product in H2SO4.
Under the microscope, I've observed elemental copper in this solidified mass. I'll be attempting the cobalt extraction soon and I'm not expecting any
extremely pure product, but I figure this is a good learning experience to see if my hypothesis works. I know there are a couple methods employed
industrially to separate Co from Cu using various organic solvents or H2S, but I don't have any of those on hand and I don't feel like forming a bunch
of hazardous H2S to deal with personally, and will also cause the neighbors to complain about the smell.
I'm fairly new to chemistry as a hobby, so my methodology, procedures and formed hypothesis of for running experiments may be way off base at times.
I started chemistry as a hobby Sept, 2017, so if I am incorrect about anything feel free to correct me and inform me why I'm wrong about something. I
just want to learn and grow, so information from others is always appreciated.


[Edited on 18-7-2018 by Doped-Al2O3-fusion]
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
The picture with the solid piece in my hand shows a bit of the copper color. The second picture shows the total contents from the reaction with
carbon. I'll be adding all the contents into a solution of H2SO4 soon.
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
I've also ran other side experiments using the solution and attempting to extract and separate the elements. I do have this nice little bag of
cobalt carbonate which I heated until it turned a nice lavender color. I performed a flame test on a small amount and I didn't see any indication of
copper which put a smile on my face.
I'm also pretty sure I formed lithium oxalate which was the first precipitate from solution. It had formed white needle like crystals, but I already
decarboxylated the contents. I did get a before shot of the crystals. I did get some nice red flames from the crystals, then was left with mostly
white ash / lithium oxide I assume. It's just mixed into the sand now and not worth taking a picture.
I've also attached a picture of the displaced copper/cobalt mix before heating with carbon.
 
[Edited on 18-7-2018 by Doped-Al2O3-fusion]

|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
I think the method I tried to separate the cobalt from the copper may actually been successful. I'm not sure if the step was necessary to heat the
contents with carbon to form cobalt carbide while simultaneously leaving the copper in elemental form and possibly reducing any copper oxide which may
have formed back to elemental copper. So, I ran two tests with one sample in HCl, and the other in H2SO4.
I placed the part of the contents from the displacement reaction using aluminum foil into a beaker of dilute H2SO4 (6mol), and left to sit over night.
I placed the contents which were reacted with carbon to reduce the copper and form cobalt carbide in HCl.--this was a blooper on my part as I meant
to use H2SO4. I had a brain fart and accidentally used HCl, only realizing my mistake about a minute later. Either way, the HCl shouldn't have
dissolved the copper. I'm not sure if it would have any effect on the CoC.
I'll be filtering the contents shortly and see what I have. The pictures here are of the H2SO4 solution. The solution using the HCl doesn't look
promising, though I realize there is a lot of carbon contamination.
 
[Edited on 19-7-2018 by Doped-Al2O3-fusion]
[Edited on 19-7-2018 by Doped-Al2O3-fusion]

|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Obviously there is a significant amount of copper sulfate in the solution with the elemental copper sitting nicely at the bottom of the flask.
Perhaps this is an alloy of copper and cobalt. I really don't know. I assume the remaining blue solution is definitely copper sulfate which formed
due to residual H2SO4 and CO2 from the air reacting with the copper/cobalt precipitate and formed copper carbonate, which would dissolve in H2SO4 to
form the CuSO4. There was obviously a significant amount of cobalt which had to precipitate from solution as the solution was dark red.
I'll see what happens as I run additional tests on the remaining blue solution. As for the HCl solution, I don't know what to expect at this point,
but I'll find out soon enough.
I'm definitely going to use the other two cells from that battery and re-do this experiment now that I know there is a lot of copper in the polymer
layers. I'll see if I can avoid dissolving copper by not using H2O2 in the initial dissolution process.
At least the cobalt carbonate I have in the little ziplock bag is slightly attracted to a magnet. I know there is no iron contamination, so if cobalt
carbonate alone isn't ferromagnetic, elemental cobalt and other cobalt complexes are. There might be enough of any number of cobalt complexes in the
bag I have labeled as carbonate.
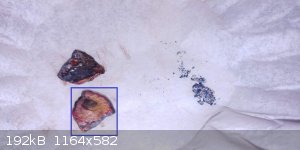
*Edit Post HCl solution filter contents compared to contents from H2SO4 solution*
The particles on the right obviously contain cobalt and this was from the HCl solution. The particles are attracted to the magnet, while the solid
chunk of mostly copper on the left show no signs at all of being ferromagnetic. I'm definitely going to re-do this experiment from scratch. The
results are at least interesting and was a great exercise and learning experience nonetheless.
[Edited on 19-7-2018 by Doped-Al2O3-fusion]
[Edited on 19-7-2018 by Doped-Al2O3-fusion]
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
I'm thinking there was quite a bit of aluminum contamination which is why I have the blue cobalt complex. It's most likely cobalt(II) aluminate.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I don't really have any comments, but just wanted to say this is some very interesting stuff. Welcome to the forum! I honestly expected this to be a
one-liner asking how to get metals out of batteries, but I'm happy to see some good experimental results and nice pictures instead! Keep up the good
work.
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Quote: Originally posted by MrHomeScientist  | | I don't really have any comments, but just wanted to say this is some very interesting stuff. Welcome to the forum! I honestly expected this to be a
one-liner asking how to get metals out of batteries, but I'm happy to see some good experimental results and nice pictures instead! Keep up the good
work. |
Thank you. I can't wait to start over and try some different techniques. I still think the carbon reduction step is worth pursuing, but go about it
differently. Chemistry is so Damn fun and intriguing.
I'll always include pictures. I document everything I do, so I have plenty to share.
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
I'm thinking about trying selective precipitation by adjusting the pH of the solution. It's pretty much the same idea as what NurdRage did with
solution to separate Mn from Fe.
In the meantime, here is a picture of a nail I placed into the HCl based solution which I dissolved the solid chunk of copper/cobalt that was roasted
in carbon. I love the dendrites visible in the picture. The copper is definitely not the typical copper color I'm used to seeing in these
displacement reactions, so I really do suspect the cobalt and copper are formed as an alloy. Some metal alloys will plate from solution such as
brass, so I'll do my research before I call this one.

|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
I realized I never posted photos of the initial solution. Here are a couple with one showing the first formation of crystals after having added the
oxalic acid. The crystals may be lithium oxalate, but then the crystals shown in the coffee filter in this reply formed earlier today after
evaporating more solution. More pinkish powder also formed which I'm assuming is more cobalt oxalate. I've got it beside the bag of what had formed
before and was heated until it turned that lavender color.
I'll check if the new needle like crystals burn red in case they are lithium oxalate. *Update--Those crystals don't burn red. It's most likely
excess oxalic acid. It left no residue and just evaporated away as white fumes.
   
[Edited on 20-7-2018 by Doped-Al2O3-fusion]
|
|
|
Doped-Al2O3-fusion
Hazard to Self
 
Posts: 99
Registered: 11-4-2018
Member Is Offline
Mood: Maniacal
|
|
Here are some pictures of what I've got so far.
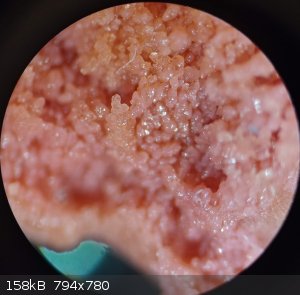
There is a shot of the last bit of cobalt sludge that's precipitated from solution. I think it's a mixture of cobalt oxalate & cobalt chloride.
There is absolutely no visual indication of copper being present in pink solid under flame test. Viewed under the microscope reveals crystal
structure, with individual crystals smoothed over from slight dissolution during rinsing process. Using cold distilled water would probably limit
yield loss.
Copper-cobalt complex was observed to be unique from pure copper obtained during the same time period and removed from solution at approximately the
same time. Both samples were rinsed with same amount of distilled water. Six initial rinses, followed by isopropyl alcohol rinse, then one last
distilled water rinse. Samples were moved upstairs away from lab area to avoid any further possible exposure to oxidizing agents. These micrograph
images were taken through the microscope eyepiece with my cell phone camera. I'll be uploading other pictures taken with my microscope mounted camera
later on due to the large image size needing reduced.
|
|
|