RogueRose
International Hazard
    
Posts: 1593
Registered: 16-6-2014
Member Is Offline
|
|
Bulk (group buy) of Ethyl Acetate ("200 proof) & maybe NaOH & KOH in buy
Well I've spent more hours trying to find ethyl acetate for a resonable price in any retail store or even onine chem suppliers from ebay to amazon. I
remember the MEK substitute (green alternative) was supposedly 100% ethyl acetate but it seems to have disappeared as fast as it showed up on the
scene, I have no idea why this would have happened. I seriously spent about 10-15 hours searching for a 1 gallon jug of MEK alternative (100% ethyl
Acetate) and never found it, and on top of that all EA was extremely expensive and very few outlets have been listing this.
I finally found 3 companies that offer a variety of EA from 90% technical grade, 99% ultra purity and "200 proof" EA of ultra high purity and all are
avabilable in 1, 5, 15, 30 and 55gal quantites and the shipping is reasonable even 3000 mile cross country!
What I want to do is find the interest in this solvent and then determin pricing based on the total interest. If there is a significant price drop
fom 30 gallons to 55 gallons from say $15/gal to $9.25/gal (30gal vs 55 gal respectively) I'd suggest we purchse the 55 gal drum and sell the
remaining on ebay and amazon in 16, 32, 64oz containers (and do multiple bottles per sale for 2 gallons, ship 4 64oz containers), and then use this
profit to help pay for the chems going to to the members of the forum. The profit from the sale of the extra amount could also be used to buy NaOH,
KOH as well and that could be shipped to people who want ti for their processing of the ethyl acetate. There are many possibilities.
What I want to know is how much ethyl acetate you are interested in in oz's, gallons, liters, etc, and a price range you will be willing to pay. Also
purity requirements are very helpful. Don't worry about placing your max price for payment b/c everyone will pay the same amount per size of EA (32,
64, 128, 5gal) so even if you say you pay $30/32oz, well the final price will probably be close to $5-$8 per 32oz, but it gives us an idea of where
people stand.
I'm also working on doing the same thing with very pure DCM and possibly methyl acetate (if there is any demand over ethyl acetate, though i'm not
well versed in the differences).
Additions to sale of ethyl acetate: NaOH, KOH & other hydroxides to make ethanol and XX acetates.
Add to this that if you are interested in buying either food grade or ACS grade NaOH or KOH, this same company sells it from technical grade, food
grade and ACS USP grade, all in prill or flake form (KOH reported to be 95% purity - other 5% is water) so there are some good options if you want to
buy good quality base to make ethanol and an K/Na acetate salt. I also am curious if the use of CaO, Ca(OH)2 or CaCO3 could be be used in place of
the K/Na hydroxides to produce a calcium acetate & 200 proof ethanol. Has anyone ever used a calcium base to neutralize ethyl acetate to make
ethanol and calcium acetate?
now I've seen that Calcium acetate has an affinity to dissolve into ethanol and make s gel-like fuel (used for heating trays on buffet tray warmers),
so idk if the calcium aspect is the best method for this, but was just curious about this aspect.
Another option would be to use Mg(OH)2 or possibly even MgCO3 mixed with ethyl acetate to make Magnesium acetate and I believe 200 proof ethanol.
This could be incorrect but i think the hydroxide would work, but the carbonate might not be workable in this case - any thoughts? What I do know is
that both hydroxide and carbonage (Mg compounds) will produce magnesium acetate.
Now I don't know what is the most beneficial acetate to make if the goal is to produce concentrated Acetic Acid or GAA, so suggestions here would be
helpful.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Sodium acetate is the best from every thing I've seen.
I like Ethyl acetate as you get two great reagents for the cost of one, Sodium hydroxide is as cheap as dirt and otc, more so then pure dry Ethanol.
Since wally world switched to methyl acetate my cost has gon up to 16 dollars for 500ml.
As for removing carbonates and impurities recrystallize the sodium acetate from methanol.
I did this by dumping it in a bucked and just dumped in room temp methanol till there was no way in hell any acetate remained undissolved.
Filtered it, then concentrated it by recovering the methanol then pouring the now super saturated methanol/acetate solution into a large dish to
evaporate to dryness.
Left me with very clean fluffy acetate, a modified method is not to super saturate it then recrystallize like normal, then wash with ice cold methanol
under vacuum filtration.
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
I buy all my common solvents (hexane, ethyl acetate, methylene chloride, etc) from Sierra Chemical on ebay. The quality is excellent and shipping
(very fast) is included in the price. The best deal for home use is to buy 1 gal quantities which comes as 4x1 quart cans making storage easy and not
having to open the whole lot at once. Sure the initial outlay is a bit high but in the long run you save money. If you recycle the solvents when you
can you save even more. I have distilled these solvents as received and 99% of the volume distills spot on for the bp. They are amazingly pure.
Among the problems you will face with large unit quantities is repackaging and LEGAL shipping. You will also need to ensure the integrity of the
material during transfer from bulk to smaller containers. Where are you going to store what are virtually commercial quantities of solvents? And you
will also need to purchase the smaller containers (cans preferably) as well as appropriate shipping boxes and properly label them for shipment.
Neither UPS or FedEx will accept a chemical shipment (beyond very small chemical samples) unless you have the proper certification or unless you feel
you can skirt the truth. Do not even try USPS as an unlicensed vendor.
You may want to rethink this scheme.
AvB
|
|
|
RogueRose
International Hazard
    
Posts: 1593
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by XeonTheMGPony  | Sodium acetate is the best from every thing I've seen.
I like Ethyl acetate as you get two great reagents for the cost of one, Sodium hydroxide is as cheap as dirt and otc, more so then pure dry Ethanol.
Since wally world switched to methyl acetate my cost has gon up to 16 dollars for 500ml.
As for removing carbonates and impurities recrystallize the sodium acetate from methanol.
I did this by dumping it in a bucked and just dumped in room temp methanol till there was no way in hell any acetate remained undissolved.
Filtered it, then concentrated it by recovering the methanol then pouring the now super saturated methanol/acetate solution into a large dish to
evaporate to dryness.
Left me with very clean fluffy acetate, a modified method is not to super saturate it then recrystallize like normal, then wash with ice cold methanol
under vacuum filtration. |
So have you found a place to get ethyl acetate? What does walmart sell as methyl acetate? I've never found it there. I have found formaldehyde
though, at about 35-45% concentration which I didn't expect and at about $20/gallon IIRC.
So are you washing sodium acetate with methanol that you get from methyl acetate? I can get methanol for about $3-4/gallon depending upon how much I
buy. IDK if there is interest in that though.
|
|
|
RogueRose
International Hazard
    
Posts: 1593
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by AvBaeyer  | I buy all my common solvents (hexane, ethyl acetate, methylene chloride, etc) from Sierra Chemical on ebay. The quality is excellent and shipping
(very fast) is included in the price. The best deal for home use is to buy 1 gal quantities which comes as 4x1 quart cans making storage easy and not
having to open the whole lot at once. Sure the initial outlay is a bit high but in the long run you save money. If you recycle the solvents when you
can you save even more. I have distilled these solvents as received and 99% of the volume distills spot on for the bp. They are amazingly pure.
Among the problems you will face with large unit quantities is repackaging and LEGAL shipping. You will also need to ensure the integrity of the
material during transfer from bulk to smaller containers. Where are you going to store what are virtually commercial quantities of solvents? And you
will also need to purchase the smaller containers (cans preferably) as well as appropriate shipping boxes and properly label them for shipment.
Neither UPS or FedEx will accept a chemical shipment (beyond very small chemical samples) unless you have the proper certification or unless you feel
you can skirt the truth. Do not even try USPS as an unlicensed vendor.
You may want to rethink this scheme.
AvB |
IDK where you got your information from UPS or Fedex about shipping chemicals but there is no problem with that unless they are oxidizers classed
above a certain hazmat rating. I called both companies and spoke to them about a long list of chemicals and asked if I needed anything special to ship
them. I only needed to follow their packaging instructions and for certain oxidizers like H2O2 above 40% I had to take a class (and some other
similar oxidizers and acids maybe). I'll have to dig up the ups & fedex shipping regs they sent me after I talked to them.
And as far as UPSP, IDK about that either because I checked out their site and talked to them as well, they had restrictions on H2O2 beyond 18% I
beleive and some chemicals needed to have a container with an outer containment layer as well which isn't an issue if you know how to pack.
[Edited on 4-30-2018 by RogueRose]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Ethyl acetate isn't the first chemical I'd go out of my way to look for. It's not super cheap, and acetone or DCM can do most of what it does. Plus,
neither of them hydrolyze under common reaction conditions. I use small amounts for TLC, but that's about it.
Ethyl lactate now, that's a solvent I really used to like. It was one of those "green" solvents that came on the market briefly, and I was able to
get it in hardware stores for like $12/quart. Dissolved similar things as ethyl acetate, but with a boiling point close to that of water. Oh, and it
smelled really nice, kind of like coconuts.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Welcome to the wonderful world of Hazmat shipping.
See below screen shot for USPS info on shipping ethyl acetate. It's 3a, flammable liquid with a flash point over 23 and under 38C. You can ship it by
GROUND ONLY, IF it can be reclassified as ORM-D, consumer commodity AND you pack it appropriately.
The flashpoint is listed variously at 24 or 25 C. Read packing requirements here:
https://pe.usps.com/text/pub52/pub52apxc_010.htm?q=Packaging...
1 gallon metal container or 1 quart non metal max. One container per package. Additional padding and a secondary containment (heavy sealed plastic
bag, plastic or metal can, etc.). Plus the outer package (probably a double wall corrugated cardboard carton), required markings & etc.
Go to Uline or a similar supplier and look at there packaging solutions, you are needing quite a few to send 55 gallons out as quart cans.
Then the actual mailing costs.
This is getting to be an expensive endeavor, yes?
SO glad I noticed this when it was still being sold at Home Depot for MEK replacer, the $14/quart they charged then is less than the postage and
packing materials will cost someone repacking bulk drums and shipping it out in quarts by USPS now.
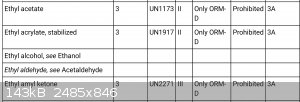
[Edited on 4-30-2018 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by RogueRose  | Quote: Originally posted by XeonTheMGPony  | Sodium acetate is the best from every thing I've seen.
I like Ethyl acetate as you get two great reagents for the cost of one, Sodium hydroxide is as cheap as dirt and otc, more so then pure dry Ethanol.
Since wally world switched to methyl acetate my cost has gon up to 16 dollars for 500ml.
As for removing carbonates and impurities recrystallize the sodium acetate from methanol.
I did this by dumping it in a bucked and just dumped in room temp methanol till there was no way in hell any acetate remained undissolved.
Filtered it, then concentrated it by recovering the methanol then pouring the now super saturated methanol/acetate solution into a large dish to
evaporate to dryness.
Left me with very clean fluffy acetate, a modified method is not to super saturate it then recrystallize like normal, then wash with ice cold methanol
under vacuum filtration. |
So have you found a place to get ethyl acetate? What does walmart sell as methyl acetate? I've never found it there. I have found formaldehyde
though, at about 35-45% concentration which I didn't expect and at about $20/gallon IIRC.
So are you washing sodium acetate with methanol that you get from methyl acetate? I can get methanol for about $3-4/gallon depending upon how much I
buy. IDK if there is interest in that though. |
Acetone free nail polish remover was usually Ethyl acetate, being the bulk of the bottle, how ever now they use methyl acetate, so no longer a usable
source here at least, was very cheap too.
The sodium acetate was dissolved into the methanol hydrate, it is then filtered, then concentrated, then cooled to precipitate the sodium acetate
crystals, these are then vacuum filtered and then washed with ice cold methanol hydrate to clean further.
This yields a very pure and clean sodium acetate.
Not as pure is to distill off methanol till it is super saturated (after filtering) then crash it out after freezing the solution, and then giving a
quick rinse of ice cold methanol.
Here 4L is 10 dollars for methanol hydrate.
|
|
|
RogueRose
International Hazard
    
Posts: 1593
Registered: 16-6-2014
Member Is Offline
|
|
I remember when I joined the forum there was a lot of complaining that MEK was going to disappear from shelves as it had just been replaced with the
"green" MEK replacement which was ethyl acetate. I think I saw this at home depot once, and then the normal MEK was back. Every other place always
had the normal MEK and never changed nor carried the alternative.
I thought it might be possible to order the MEK replacement online but it seems impossible to find, especially in the gallon, there are quarts here
and there buy at $19-25 for a quart, I'll pass.
As for nail polish removers, same thing, Some was EA about 3-4 years ago but now I can't find it anywhere. I've looked at every grocery or drug store
I've gone to just out of curiousity. Acetone reigns kind followed by methyl acetate then polyethelene carbonate (I think that is the correct
compound), which I have heard can be an interesting compound.
Even looking for EA online like on ebay can be hit or miss, sometimes there are a few listings, sometimes there are none (for months - 3-6 months at a
time).
I also checked PPG, Sherwin williams and Benjamin Moore, and none of them carry it now (so they say over the phone), which is odd b/c many of their
products contain it as a %, the same with Toluene, "don't have it" yet if you ask in store, they often have it in qt, gal and 5 gal at the store!
I can't seem to wrap my head around why it is difficult to find. There are PLENTY of sources of ethanol for less price, often 100% drinkable from the
bottle at 95% purity. I've found EA is a good solvent for seperating some salts where one is insoluble while another is highly soluble in EA, so I'd
like to get some to work with but I'm not spending $80-100 per gallon for it.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Atm there is still ethyl acetate nail polish remover but it is 4 dollars for a pathetic yield of 100ml or so, only reason I get it is for making
sodium acetate as atm still cheaper then just ordering it due to the fact I end up with pure ethanol as well for my troubles. but with the bioflame I
get much larger ethanol cheaply so even that is don, but I am distilling out 500ml at 16 dollars  pricy but I don't use it much as of yet, just want 500ml on the shelf. pricy but I don't use it much as of yet, just want 500ml on the shelf.
|
|
|
|