Σldritch
Hazard to Others
  
Posts: 310
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Beryllium from Beryllium Oxide
I have several magnetrons laying around as well as a lot of Magnesium so naturally i want to try to extract Beryllium metal from the magnetrons.
Due to Beryllium's toxicity i do not want to dissolve the Beryllium if at all possible nor do i want to crush it. The safest option i can think of is
fusing Beryllium Oxide, a flux and Magnesium metal.
The hardest part for me is the flux; Sulfates will not work due to their reaction with Magnesium leaving Sodium Chloride the next most obvious choice
but due to the low boiling point of Beryllium Chloride (BP:755 K) it seems extremely dangerous. Beryllium Fluoride (BP: 1,442 K) is considerably
better but still fairly low considering the temperatures readily achieved with thermites and using Sodium Fluoride for safety seems somewhat
counterproductive.
Therefore my questions are:
1. Does anyone have any experience with this?
2. Does magnesium and Beryllium form alloys or intermetallic compounds?
3. What flux should i use?
|
|
|
unionised
International Hazard
    
Posts: 5142
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
"The safest option i can think of is fusing Beryllium Oxide...".
Not an easy option- Mpt is about 2500C
The big problem is keeping the Be under control. If it's hot + exposed to air it will catch fire and give off very toxic smoke.
I really can't think of a good way to do this.
|
|
|
Σldritch
Hazard to Others
  
Posts: 310
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
I don't think the Beryllium Oxide will melt, but Beryllium is a fairly strong Lewis acid and can form low-melting complexes such as Sodium
Tetrafluoroberyllate (MP: 575 degrees Celsius).
|
|
|
unionised
International Hazard
    
Posts: 5142
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
The toxicity is still the big problem.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Per atomistry.com on beryllium (http://beryllium.atomistry.com/):
"Metallic beryllium can be prepared by electrolysis, by the reduction of the oxide with magnesium, of the chloride with sodium, and in other ways. It
is a white metal, which is still more stable to moist air than magnesium, and decomposes water only slowly even when heated. It is readily dissolved
by dilute acids, with evolution of hydrogen, and passes thereby into the ionic state. "
|
|
|
violet sin
International Hazard
    
Posts: 1497
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
So are we just taking it for granted that microwave magnetrons are FOR SURE composed of beryllia and not just alumina? Do we have any composition %
breakdown, or just the ravings of various online posters from YouTube and such? I looked several times for documentation and even e-mailed some
manufacturers, to no avail. Was actually hoping to inspect innards after grabbing magnets. Never felt the reward was worth the risk in beryllia
territory.
I have some old Ar-ion laser heads which for sure do have beryllia, as per their visible warning/danger labeling. Never seen that on magnetrons, but
the "do not open, no user serviceable parts" could be a blanket warning. Still, should the device short out and draw a heavy arc whilst fuming the
air with ceramic smoke,.. a warning would have to be present if accident could poison/cancer your whole building.
I would simply love to find a landmine map of sorts if there is a published list of manufacturers using beryllia ceramic compositions, and those not
using it.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Violet Sin is correct there is no beryllium in the domestic microwave magnetrons. There is no reason to use an expensive and potentially toxic ceramic
when a cheap non toxic one will do.
I have attached a pic of four ceramic items I have. The two on the left, one pink and one white, are tig nozzles. The two items on the right again one
pink and one white are the ceramic ends of a magnetron. The wires are connections to the filament The filaments usual break during disassemble as they
becomes very brittle and fragile in old magnetrons.
Many alumina ceramics are pink even alumina grinding wheels.
Correction: the white nozzle is a plasma cutter nozzle.
[Edited on 18-4-2018 by wg48]
|
|
|
Σldritch
Hazard to Others
  
Posts: 310
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Damn, you are probably right, i have only heard it from various unreliable sources on the internet. I would confirm by measuring the density but i
could not dismantle it far enough safely, could you?
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Σldritch  | | Damn, you are probably right, i have only heard it from various unreliable sources on the internet. I would confirm by measuring the density but i
could not dismantle it far enough safely, could you? |
Yes its always good to do a test for more confirmation.
Most of the metal can be removed with a small hacksaw or by turning in a lathe. The rest can be dissolved in HCl. The copper parts take a long time
as it requires oxygen. Nitric acid would be quicker and may dissolve the metallisation that was initially put on the ceramic to enable the metal parts
to be joined via brazing. HCl does not.
I think I have some parts that have most of the metal removed except for the metallization but I do not recall if they are pink or white.
I will check and report back.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
I did not have a pink ceramic insulator from a magnetron without attached metal so I made one by dissolving the metal and removing the acid resistant
wires which I assume are tungsten or molybdenum metal wires. The ceramic is shown below.
I calculated its volume from its dimensions assuming the two raised areas in the pic had the same volume as the holes and nick. Then measured its
mass. The density calculation is shown below.
The calculated density is 3.654 g/cm^3 which is consistent with an aluminium ceramic density of 3.75 to 3.95 g/cm^3
and not a BeO ceramic density of 2.85 to 2.92 g/cm^3.


The item to the right of the pink ceramic is a 10g calibration mass I used to check the scales. The dimensions were measured with digital calipers
with a 0.01mm resolution.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Nice Maths !
I had to check it 3 times as my first result was a bit off.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Perhaps you will like the following screen shot of part of my analysis of a temperature control system.
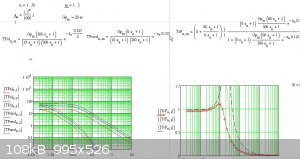
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Awesome !
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
I now have two ceramics from magnetrons the pink one previously discussed and a white one they both have a mass of 13g and almost the same dimensions
within a few 0.01mm and hence almost identical densities compatible with the density of alumnina.


|
|
|