K1996
Harmless

Posts: 7
Registered: 11-2-2018
Member Is Offline
Mood: No Mood
|
|
Aromatic aldehydes using Gattermann Koch Reaction
Hey everyone, brand new user here.
So I’m an undergrad chemistry student, I’m trying to design a set up for a reaction but the materials are kind of expensive so I don’t have much
room for failure. My compound is a metallocene, meaning it’s a metal with two cyclopentadienyl groups sandwiching it. I’m trying to formylate one
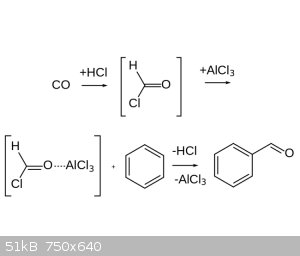
of the rings and I’ve settled on the Gattermann Koch Reaction which seems the easiest to do. The reactions requires CO to be reacted with AlCl3
which supposedly forms a formyl chloride intermediary and that follows a Friedel-Craft-esk mechanism which then attaches an aldehyde group to the
aromatic ring. So my question is how to set up the reaction, I’ve read around and the set up people seem to use is to bubble CO and HCl gas into a
flask containing the AlCl3, cuprous chloride (for some reason, I couldn’t find its role in the reaction), and the aromatic compound all disolved in
ether or nitrobenzene.
So my thoughts are to get the CO from Formic acid by adding sulfuric acid drop wise and having a tube go from that flask to the reaction flask, my
doubts here is if that’ll produce enough CO to drive the reaction or even how much formic acid to use or how fast to add the H2SO4 since there’s
also a risk of using too much and formylating more than once or formylating both rings in the sandwich molecule. The HCl I’m not sure how to get it
in gas form, wouldn’t the same effect be achieved with some concentrated hydrochloric acid? I’m also thinking of adding a NaOH trap to catch and
destroy any carbon monoxide and HCl gas that escapes.
Honestly any input to help me optimize this design would be greatly appreciated, I really want to go through with this reaction but my mentor is
currently occupied with other projects so I want to approach them with most of the work done so it’s just a matter of going through with the
reaction, and like I said the reactants are a bit on the expensive side so I want to waste as little as possible.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Vilsmeier conditions would be easier if they're compatible with your substrate.
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
@K1996, is it mandatory for your work to use Gattermann Koch Reaction to do the formylation? did you think about do this formylation by other ways?
I'm thinking about two ways to do this formylation in an OTC way:
first, via aldimine followed by hydrolysis like this:
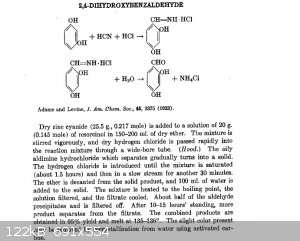
Or second, via glyoxilic acid followed by decarboxylation of the alpha-hydroxy-acid formed with sodium hypochlorite (bleach), like this:
"Glyoxylic acid is reacted with benzene, in the presence of sulfuric acid in an amount of 0.1/1mol, preferably 0.3/0.8mol based on 1mol of the
glyoxylic acid, and acetic acid in an amount of 0.5/10mol, preferably 4/8mol, at 20-80°C, preferably 40-80°C, to give mandelic acid.
Glyoxylic acid as the starting material is available as an aqueous solution usually in a concentration of about 40-50%. After the above-mentioned
reaction is completed, the resultant solution is separated into a benzene layer containing the titled compound and an aqueous layer. An aqueous alkali
salt is added to the benzene layer, and the extracted mandelic acid in the form of an aqueous alkali salt is then deposited with an acid to give
mandelic acid as a precipitate."
"Acta Chem. Scand. B38(4), 343-344 (1984)
Oxidation of alpha-Hydroxy Carboxylic Acids with Sodium Hypochlorite
General Procedure. A solution containing 10 mmol of the alpha-hydroxy carboxylic acid in 25 ml of diethyl ether was cooled in an ice-bath. Then 40 ml
of a commercial bleach solution (containing 4.2% sodium hypochlorite and 0.5% sodium hydroxide), 22 mmol, was added over a 3 min period. The reaction
mixture was then allowed to warm up to room temperature for 2-3 h. The organic layer was separated, washed with water, dried over anhydrous magnesium
sulfate and the solvert evaporated. The crude product was purified by molecular distillation or recrystallization."
[Edited on 11-2-2018 by Chemi Pharma]
|
|
|
K1996
Harmless

Posts: 7
Registered: 11-2-2018
Member Is Offline
Mood: No Mood
|
|
I had already proposed that reaction to my mentor, but they don’t like working with toxic compounds (or probably more accurately doesn’t want us
undergrads to work with them) and wasn’t fond of using POCl3.
|
|
|
K1996
Harmless

Posts: 7
Registered: 11-2-2018
Member Is Offline
Mood: No Mood
|
|
@Chemi Pharma it doesn’t have to be Gattermann Koch, it’s just the one that I’ve found. I like the yield on your second method. I’m almost
sure my mentor wouldn’t approve of the cyanide but I’ll consider it too. Thanks!
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Your supervisor takes issue with POCl3 but not with CO? I don't know what to say...
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2788
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Is acetic formic anhydride applicable to aromatic substrates? This off-the-shelf reagent can perform formylations as though it were an acyl halide, if
you can buy it that is. Probably a "yes" since your professor is helping you.
|
|
|
K1996
Harmless

Posts: 7
Registered: 11-2-2018
Member Is Offline
Mood: No Mood
|
|
To be fair I haven’t told them about the CO yet, I was thinking of adding an NaOH and 3% peroxide trap to destroy any CO and HCl gas.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
CO is one of the worst. POCl3, on the other hand, is not too bad.
http://orgsyn.org/demo.aspx?prep=cv4p0539
[Edited on 11-2-2018 by zed]
|
|
|
K1996
Harmless

Posts: 7
Registered: 11-2-2018
Member Is Offline
Mood: No Mood
|
|
I know, i actually had the whole synthesis for the compound I wanted, I looked at the safety data for POCl3 and it was pretty much only bad if you
swallowed it, apart from its odor which is supposedly pretty bad or something. But my mentor pretty much completely shut off from using it. My
thoughts are that if I approach them with a well constructed method for the reaction with no possibility of leakage as well good yield they’ll
probably say yes, but for all I know it might go the same as my previous proposal. I chose the CO method because it was the easiest/straightforward
method I could find, I don’t want a many step synthesis because I need the yield to be good because the starting materials are expensive and the
product of this reaction is actually the starting material for the synthesis I actually need for my research.
But I’m not exactly attached to this method if anyone has better method I’m seriously all ears.
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
@K1996, about the aldimine idea, note that hydrogen cyanide is generated only in situ, by the reaction of zinc cyanide and HCl gas. Only a little
amount of the free form will be detected and possible escape from the vessel. The rest will be consumed by the reaction.
In any case, I'd rather to do this reaction inside a fumehood anyway. Every University Chemistry Lab have one or more fumehoods, isn't it?
one more note: I think zinc cyanide can be subsituted by copper cyanide, without screw up the reaction. Copper cyanide is easier to find than the zinc
salt at chemical suppliers and, at least here where I live, it's not under control at all.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
You're unlikely to find less dangerous conditions. In terms of generating a gaseous reagent ex-situ, its doable but I wouldn't recommend it,
particularly to an inexperienced researcher like yourself. Considering the Gatterman Koch requires HCl and CO, thats two gas generators that you're
going to have to set up, adjust and maintain appropriate flow for, and thats before you even start considering whats happening in your actual reaction
mixture or how you're going to scrub the exhaust flow. Using a simpler method reduces the number of variables to troubleshoot should things not go to
plan.
I also noticed you're mentioning that the starting materials are expensive - test the reaction first on small scale (tens of milligrams) and have a
plan of how you're going to check the progress of the reactuon. Also consider what you can do if it doens't appear to work on first instance. For this
reason alone it is worth avoiding gaseous reagents - they do not typically scale down well, and certainly not without suitable equipment.
If the aldehyde is to be reacted in a subseqeunt step, then perhaps there is an alternative approach to this product? I don't know what you're
planning on doing, or if you're able to disclose it publicly, but it is something for you to consider.
In terms of formylation reactions that are operationally simple, the Vilsmeier-Haack reaction probably takes top place. Also worth considering is the
Rieche formylation, which uses dichloromethyl methyl ether and SnCl4 (typically).
The use of formic-acetic anhydride might be possible too - actually there was a paper published very recently using carboxylic acids in TFA/TFAA to
perform FC-acylations - not sure if formic acid is applicable but worth looking into: https://doi.org/10.1016/j.tetlet.2018.01.026
There's also this older paper that I'd trust a lot more, but again I do not know if it is applicable to formylation with formic acid: https://pubs.acs.org/doi/10.1021/op9700136
[Edited on 12-2-2018 by DJF90]
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
What about Duff reaction or Reimer-Tiemann reaction?
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Duff reaction is something interesting and can be done easily for academic purposes, but Reimer-Tiemann reaction is appropriated only for phenols, not
for unsubstituted aromatics, like in this case.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Why do you want to formylate it, as opposed to, say, acylating it (which would be far easier)?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. At a big U., you shouldn't have trouble acquiring POCl3, but you might. Ask your mentor, if acquisition is the problem. The guys here, have a
simple method for producing small quantities from Phosphorus Pentoxide.
|
|
|
K1996
Harmless

Posts: 7
Registered: 11-2-2018
Member Is Offline
Mood: No Mood
|
|
My mentor did say that an acylation is also acceptable but the molecule I want to use is analogous to a previous molecule my lab made, what I want to
test is what happens when I change the metal center compared to that previous compound, so I want the legand to stay the same so I don’t introduce
any unknown variable that skews the result one way or the other. Acylation so far is my last resort.
@Outer from what I could find, the duff reaction also needs phenols.
@zed I don’t think it’s inability to acquire it as much as unwillingness to work with it.
@DJF90 the rieche reaction seems promising, The papers seem interesting but I’ll read those in the morning it’s pretty late now.
|
|
|
K1996
Harmless

Posts: 7
Registered: 11-2-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Chemi Pharma  |
In any case, I'd rather to do this reaction inside a fumehood anyway. Every University Chemistry Lab have one or more fumehoods, isn't it?
|
Usually yeah, but for the sake of anonymity let’s just say my university went through a thing and our labs aren’t in their best conditions,
especially us the not super funded research groups. That’s also why my mentor is being hesitant with what sort of reactions we do.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by K1996  |
My mentor did say that an acylation is also acceptable but the molecule I want to use is analogous to a previous molecule my lab made, what I want to
test is what happens when I change the metal center compared to that previous compound, so I want the legand to stay the same so I don’t introduce
any unknown variable that skews the result one way or the other. Acylation so far is my last resort. |
Makes sense, but if you want to do a direct comparison, you could also make the acyl analogue of the previous molecule....
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Chemi Pharma  |
Duff reaction is something interesting and can be done easily for academic purposes, but Reimer-Tiemann reaction is appropriated only for phenols, not
for unsubstituted aromatics, like in this case.
@Outer from what I could find, the duff reaction also needs phenols. |
It depends of reactivity of rings of your metallocene, activated rings would react.
Or see other possible reactions:
1. Chloromethylation by CH2O+HCl to Ar-CH2Cl, then Sommelet reaction with the same hexamine to an aldehyde, or oxidation by DMSO (Kornblum oxidation).
2. Oxymethylation by formaldehyde to Ar-CH2OH, then oxidation.
3. Mannich reaction to Ar-CH2-NR2, then oxidation by H2O2 to N-oxide, then dehydration by Ac2O to an aldehyde.
4. Reaction with orthoformate (see example: doi.org/10.1080/00397910008087203)
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Umm. What are you going to do with that aldehyde, once you have it?
If it is a waystop to synthesising another functional group, it may be possible to implant the whole structure directly.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Mixed anhydride of Formic/Acetic acid?
Now that, sounds interesting! Which acid is more likely to predominate in the attack?
|
|
|