jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
3,4-dimethylaminorex
has anyone looked at the pharmacology of this anolog?
In this study they indicate the 4s,5s isomer as having dopaminergic nuerotoxicity and that it can induce fatal seizures that tells me this is very
potent. They also indicate the maximum tolerable dose in rats is 5mg/kg 4s,5s, and 30 mg/kg for 4r,5s, where in the case of 4-methylaminorex it's
15mg. for both isomers (both cis and trans isomers).
Since a well known reaction pathway produces only a trans isomer it appears that it's possible to synthesize the "safer" isomer.
http://www.erowid.org/references/refs_view.php?A=ShowDocPart...
another reference I have is Buur, A.: Bundagar, H. arch. pharm., chem. sci. ed., 1987, 15, 76
Yuong-harvey, J.A.; Rae, I.D.: pitman, I.H. int. J. Pharm. 1986, 30, 151
Chalina, E.; Dantchev, D.; Georgiev, Mitova, k. Arch. Pharm, 1986, 319, 598
The article referenced on it's chemistry is
Poos, G.I.; Carson, J.R.; Rosenau, J.R.; Roszkouski, A.P.; Kelly, N.M.; Mcgrowin, J. J. Med. Chem., 1963, 5, 266
prepn. of the oxazolidine-2-imines from ephedrine:
Fodor, G.; Koczka, K. J. Chem. Soc., 1952, 850
Noggle, F.T. Jr.; clark C.R.; and Deruiter, J. J. AOAC int., 1992, 75, 423
[Edited on 21-2-2007 by jon]
[Edited on 21-2-2007 by jon]
[Edited on 21-2-2007 by jon]
[Edited on 21-2-2007 by jon]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I'm going to be making the (3,4 MD)-4-MAR. I am sure its covered by existing patents but I have not read reports of its effects. I am very curious.
Will it be psychedelic? Weaker stimulant? Anorexic? None of the above (most likely)? Or could it be a nootropic? Are you going to make the 3,4
dimethyl? Is it safe to do so? I hear the trans is technically unfettered with fed sores.
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
the poos article I sent you would explain this they lose much of their activity when methoxylated in the 4-position I would'nt even bother.
when the 4-postition posesses an electron withdrawing substituent it's activity increased.
and technically the trans isomer is not scheduled but a weak circumstantial case has been made in the past and, that's all it takes. you see the law
is grey when it should be black and white and vise versa.
[Edited on 22-2-2007 by jon]
|
|
|
MeSynth
Hazard to Others
  
Posts: 107
Registered: 29-7-2011
Member Is Offline
Mood: http://www.youtube.com/watch?v=5ZltqlVuDIo
|
|
found it
Found this on drug-forums after a google search for this neurotoxic isomer... No refs are provided. I haven't searched for them and probably won't.
(this isn not the whole post)
--------------------------------------------
chemman added 6 Minutes and 10 Seconds later...
CHEMISTRY

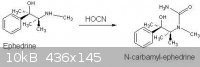
N-Carbamyl-ephedrine .Potassium cyanate (2 g., 0.025 mole) was added to ephedrine hydrochloride (5 g., 0.025 mole) in water (25 ml.), and the solution
then refluxed for 24 hours, during which a small amount of oil separated, and cooled in ice-salt. The dried, white plates of the urea (3 g; 57.7%)
were crystallised from ethyl acetate and then had m.p. 126-127 deg C.

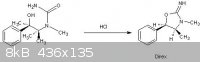
trans-2-Imino-3,4-dimethyl-5-phenyloxazolidine hydrochloride:
A solution of N-carbamyl-ephedrine (1.56 g., 0-075 mole) in water (24 ml.) and 2N-hydro-
chloric acid (15 ml.) was refluxed for 3 hours; when the clear solution cooled the crude oxazolidine hydrochloride was obtained. This was purified by
conversion into the base (use Na2CO3 or K2CO3) which was extracted with benzene. The solvent was removed under vacuum from this, and the residue
(direx freebase) converted into the hydrochloride (1-9g.; 84%), m. p. 225-229.
You can also use freebase to smoke or oral consumption.
--------------------------------------------------------------
|
|
|
spunonrun
Harmless

Posts: 2
Registered: 6-9-2011
Member Is Offline
Mood: No Mood
|
|
http://www.drugs-forum.com/forum/showthread.php?t=83865
[Edited on 7-9-2011 by spunonrun]
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
The N-Carbamyl-ephedrine is a urea. Look at the carbonyl flanked by two nitrogens.
|
|
|
overload
Hazard to Self
 
Posts: 66
Registered: 9-7-2011
Location: USA
Member Is Offline
Mood: miserable fat slave
|
|
Yeah thats where I got it. The other stuff is pretty useless and that's why I did not add the link. Does anyone have any idea if this really works?
Spunonrun looks like he already knows....
|
|
|
overload
Hazard to Self
 
Posts: 66
Registered: 9-7-2011
Location: USA
Member Is Offline
Mood: miserable fat slave
|
|
Aminorex has more potential as an anerectic.
|
|
|
roamingnome
Hazard to Others
  
Posts: 363
Registered: 9-9-2006
Member Is Offline
Mood: No Mood
|
|
stumbled on this "potent" paper
its seems that the methyl group of ephedrine prevents the double bond from being in the oxazoline ring so there is a imine type nitrogen ...whereas
in methylaminorex you see a regular amino group. unless bond inversion is readily happening this may describe 5-HT2B antagonism ?
4dmar needs hydrogenation but not overly so
i am referring to page 851 fig (VIII)
prepn. of the oxazolidine-2-imines from ephedrine:
Fodor, G.; Koczka, K. J. Chem. Soc., 1952, 850
Attachment: dimetyloaminorex.jcs.1952.850.pdf (566kB)
This file has been downloaded 1069 times
[Edited on 9-11-2011 by roamingnome]
[Edited on 9-11-2011 by roamingnome]
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
mdMAR is not active 
dimethylAR or the 3,4methylaminorex is active at about 1/3 as potent as MAR.
I am suprised you do not know this it is very easy to get there.
aminorex is dangerous
and all of the MAR family are not selective enough and burn holes in your
head which lead to psycosis which can bee seen from MRI scans which are
freely available on the web.
there are better things to study than MAR.
it may be worth money but honest so are so many other things that dont
leave a shit hole for your kids to grow up in.
jon look into syringaldehyde into coke you might even save some
of our forests in the process.
it has been done by quite a few believe it or not  . .
still this substance is not what I would call progressive
development.
but hey its better than MAR substances.
safer is not dosage relative in my eyes.
its living after using.
[Edited on 11-11-2011 by Ephoton]
e3500 console login: root
bash-2.05#
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
yeah i can say you don't want to mess with these class of drugs i did and the end result was violent cconvulsions and sky high blood pressure.
the pressure was so great it was as if my head was about to explode.
dangerous stuff.
Give me librium or give me meth!
Patrick Henry....
|
|
|
Polverone
Now celebrating 21 years of madness
|
Thread Split
11-12-2011 at 14:09 |