tizrhf
Harmless

Posts: 8
Registered: 2-2-2007
Member Is Offline
Mood: No Mood
|
|
The aromaticity of halonium ions
Hello! I'm new on this forum. Last week i came across an explanation for the known stability of halonium ions. It said that these kind of ions have
aromatic character (2 pi electrons). Chlorine, being the most electronegative of the halogens involved in forming halonium ions would destabilize the
aromaticity with it's strong inductive effect, so this would explain why the chloronium ions are the most unstable from the series. Now the thing that
bothers me is that I don't understand how this halonium ions are aromatic (those 2 electrons come from the halogen) because I can't see any pi-pi or
n-pi conjugation there. The halonium ions derived from alkynes have a double bond in it, which creates a great tension and instability. But their
instability is also related to the fact that they are antiaromatic (that is, they have 4 pi electrons). But that confuses me a lot because that ion
looks just like a 2-electron cyclopropenilium ion. Of course there, the number of pi electrons (2) and the conjugation is very obvious. I would be
very grateful if someone could clarify this situation. Thank you very much.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I never heard of such an explanation for the halonium species. Do you mind giving the reference to the where you read about it?
|
|
|
tizrhf
Harmless

Posts: 8
Registered: 2-2-2007
Member Is Offline
Mood: No Mood
|
|
I am sorry. I'm from Romania and it's written in a romanian book, so i don't think you will ever get the chance to read it. But before reading it, a
friend of mine told me about the same thing, which was explained to him by a professor. So i'm pretty sure it is true, but still i don't know why. I
was surprised at first about this explanation, but since there are two sources (that can be trusted) which tell the same thing, iguess we can assume
it's true.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Scientists are skeptics. Cite the Romanian book, scan the chapter, post it here, someone will be able to translate.
Lacking that I'd suspect a case of aromatic balonium.
Not trying to hurt your feelings, and welcome to the forum, but we are wondering if you are having an April Fools joke on us a little early.
[Edited on 18-2-2007 by Sauron]
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
I understand some Romanian. Post it.
damnant quod non intelligunt
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The question to ask yourself is, what is the essential meaning of aromaticity (in contest of organic chemistry) and what is the smallest compound that
can possibly be regarded as aromatic?
Just what exactly does a halonium ino look like?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Definitions of Halonium ions from Web:
1.
Ions of the form R2X+, where X is any halogen (X = Br+, bromonium ions; X = Cl+, chloronium ions; X = F+, fluoronium ions; X = I+, iodonium ions).
They may be open-chain or cyclic.
GNOC Recom. R-5.8.2. E.g.
2. Narrower definition:
Halonium ion -- a three-membered cyclic cationic intermediate in which a halogen bears formal positive charge; formed by the reaction of X+ with an
alkene; important for chlorine and bromine (10.2)
Note that both structures below meet first definition while only the three membered ring meets second.
I remain neutral to both definitions but propose we use one or both, arguendo, till something better comes along.
So the question is: see any aromaticity? Because I don't.
Not according to Huckel. I don't see any pi bonds.
[Edited on 19-2-2007 by Sauron]

|
|
|
tizrhf
Harmless

Posts: 8
Registered: 2-2-2007
Member Is Offline
Mood: No Mood
|
|
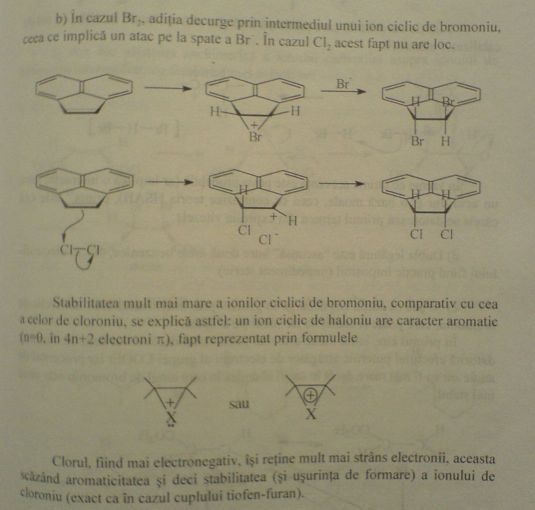
So here is the exercise in that book i was talking about. It explains the stereoselectivity of the halogen adittion of bromine and chlorine to that
double bond. It says:
"In the case of Br2, the addition goes through a cyclic intermediate, the bromonium ion, which in turn implies a back-attack of Br-. This doesn't
happen in the case of Cl2". This is above the mechanism representation. Below it:
"The greater stability of the cyclic bromonium ions, compared to that of a chloronium ion can be explained in this way: the halonium ion has aromatic
character (n=0, 4n+2 pi electrons), which is represented by the formulas:"
(There are the two formulas there - i cannot see ani pi conjugation there, you are right sauron, but nobody said anything about pi conjugation, we can
suppose it's something else right there).
Below the two representations:
"Chlorine, being more electronegative, keeps his electrons tighter, this causing a decrease in aromaticity, and hence, the stability (and the ease of
formation also) of the chloronium ion (just as in the case of thiophene and furan)".
This is the exact translation. I was quite confused when I saw it first, but i also found out that halonium ions of alkynes have antiaromatic
character (4 pi electrons, that is two from the halogen and two from the double bond. Remarkably, this ion looks just like cyclopropenilium, but it
isn't actually the same thing. So i guess there is no pi conjugation involved).
Thank you very much for studying this aspect and helping me.
[Edited on 19-2-2007 by tizrhf]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I am having eye problems and will have to clip that drawing to my HDD and edit it for contrast before I can read it...
But you are talking about a transition state, aren't you? What's the life expectancy (say, half life) of a bromonium ion (the most stable of them)?
Can they be trapped and observed? Or is their existance inferred as an explanation for the demonstrable behavior of the halogen addition to double
bonds? (vz Markovnikov I suppose.)
Has this been subjected to ab initio and/or semiempirical computational analysis like Gaussian, MOPAC, AMPAC etc? Those handle input files for this
sort os specie. In fact you could use them to determine the transition state and if they predict a halonium ion you have independent confirmation of
this hypothesis.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
There are some basic rules a compound must conform in order to be aromatic. One is that the number of Pi electrons must be in accordance to the
Hückel's rule (2+4n where n=0,1,2…). The trimembered halonium species can mobilize 2 Pi electrons from the unshared electron pair of the halogen
(n=0). But the problem is that what is depicted as a resonance stabilization of the cation is not a true resonance since a resonance only involves Pi
bonds while in the halonium species it is the sigma bonds that stabilize the charge (it is more like hyperconjugation). So the situation is not like
in the aromatic cyclopropenyl carbocation from which the analogy is obviously taken.
I'm quite skeptic of such claims of halonium aromaticity, especially in the view that I never heard the halonium species are any more stabile than
what can be explained by the hyperconjugative stabilization.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
tizrhf
Harmless

Posts: 8
Registered: 2-2-2007
Member Is Offline
Mood: No Mood
|
|
It's been proved that halonium ions truly exist. Chloronium ions, actually, they do not form because they are too unstable, with the reasons shown
before, but very hindered bromonium ions that do not permit further attack of the negative bromine anion can be at least detected spectroscopically,
maybe isolated. This picture taken from my clayden will clarify.
[Edited on 20-2-2007 by tizrhf]

|
|
|
tizrhf
Harmless

Posts: 8
Registered: 2-2-2007
Member Is Offline
Mood: No Mood
|
|
No, halonium ions ARE NOT transition states !!!
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Indeed, hey are reaction intermediates. And chloronium ions do exist. But your original question is still unanswered.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Well at least my questions got answered.
If te stuff lasts long enough to be characterized then of course it isn't a transition state, nor is it merely hypothetical, at least in the case of
bromonium and chloronium.
But I still share Nocodem's dubiousness about aromaticity. Having only one or some of the requisite requirements for aromaticity is like being a
little bit pregnant. And coining a term like quasiaromatic (and no you didn't) would be counterproductive, as it would merely muddy the water,
blurring the distinction.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Oh, and you ought to edit that last image for size, it is distorting the page and vulture has a proclivity to delete such.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
You in 800x600? The image is no problem for me.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
That image is 1017 x218 pixels.
And yes I am in 800 x 600.
It is causing the text to flow off screen requiring one to use horizontal scroll and THAT is what vulture does not want.
All the fellow has to do is edit the image down to fit on the page. I think vulture mandates 600 x 400 or 640 x 480, I'm not sure, but 1017 is too
wide.
|
|
|
tizrhf
Harmless

Posts: 8
Registered: 2-2-2007
Member Is Offline
Mood: No Mood
|
|
I edited the image, thanks for warning me about it. Well, I know that my question still remains unanswered and i know it is a very tricky question,
but my intuition tells me it's right, i trust the guy that told me about it, though he couldn't explain anything about it. What do you think i should
do? Do you know somebody who might clarify such things?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
There are a lot of fine chemists on this forum and I'm sure they will step up to the plate.
Thanks for editing.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by tizrhf
Well, I know that my question still remains unanswered and i know it is a very tricky question, but my intuition tells me it's right, i trust the guy
that told me about it, though he couldn't explain anything about it. What do you think i should do? Do you know somebody who might clarify such
things? |
If the threemembered halonium cations would be aromatic then they would make the first case of an aromatic system without any Pi bond at all. I vote
against its aromaticity.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I think I said as much way up the thread. So I agree with Nicodem and I also do not believe that the three membered ring halonium ion, sans pi bond,
can be regarded as aromatic.
Thumbs down from me (again.)
Now, suppose that the bond the halogen was adding to was acetylenic, and the three membered ring would thus have a pi bond across the 2,3 carbons. I
think there you could make a stronger case for aromaticity. What say you, Nicodem?
|
|
|
tizrhf
Harmless

Posts: 8
Registered: 2-2-2007
Member Is Offline
Mood: No Mood
|
|
I already said, halonium ions formed from alkynes have antiaromatic character (4 pi electrons).
|
|
|
tizrhf
Harmless

Posts: 8
Registered: 2-2-2007
Member Is Offline
Mood: No Mood
|
|
I think i got it. Well, the halogen atom there is actually a hypervalent positive center. This makes one of his lone pair's orbitals of high p
character. The orbitals on carbon also have high p character. We know that we can write the halonium as a carbocation ( resonance structure). This
makes the conjugation all right, the 2 electrons in the high p-character orbital of the halogen can interact freely with the orbitals on carbon. In
benzene and in any other aromatic syste, we have interactions between p orbitals. It,s the same here, but of course i'm not talking of the same gain
of stability, because the interactions in the halonium ions are much smaller than in the benzene ring. We have two electrons, so Huckel's rule is
obeyed, we have continuos interaction between 3 p orbitals, the halonium is planar. these would all mean one thing: AROMATICITY.
What do you say about my "theory" ?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I'd say it's more of your hypothesis because it hasn't matured into a theory yet.
I think if in fact aromaticity were evident then there would be more stability shown than there is. As it is, boromonium ions are more stable than
chloronium and then only when highly hindered, right?
So, I would say that while halonium ions have some of the aspects associated with aromaticity, they don't have all of them, and in particular, the
very weakness of the interactions you describe argues against any claim of aromaticity.
Sorry. No cigar. Your argument, it seems to me, is that the hypervalent halogen could interact with the two carbons (thus three pi orbitals) but that
does not demosntare that such interaction actually takes place.
Why don't you take my earlier suggestion and crunch this through Gaussian or MOPAC or GAMESS or whatever MO package you like and the data dump will
tell you in no uncertain terms whether or not the molecule is aromatic, or not.
Do your structure input file rigorously, and make it available along with the output, and any computational chemist will be able to verify that the
input and the results are meaningful (or identify errors.)
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I think this arose from a poor choice of words in the text. Some aspects of aromaticity aren't aromatic. Aromatic has a specific meaning having the
Huckel number of Pi electrons. As Nicodem points out, sigma bonding is evidently involved in hyperconjugation? Maybe conjugated-like stability is
better.
Question? From the bridge, which way does the first Halo ion go? Markovnikov or anti?
[Edited on 22-2-2007 by chemrox]
|
|
|