| Pages:
1
2 |
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Reduce cinnamaldehyde to hydrocinnamaldehyde
Hey guys,
I am trying to reduce cinnamaldehyde to hydrocinnamaldehyde, and have several reducing agents at my disposal, including several metals, hydrazine,
sodium dithionite, NaBH4, and Red-Al, etc.
I want to protect the carbonyl group, and not have it reduced to an alcohol. I know that NaBH4 will prefer reduction of the a,b-unsaturated conjugated
double bond since it is a softer nucleophile, but since its an aldehyde and not a carboxylic acid, the aldehyde will get reduced to an alcohol in the
second step.
Is there a way to protect the aldehyde while still being able to reduce the conjugated double bond?
I have some ideas, and they include sodium dithionite under PTC conditions. I have found a paper where they use a PTC in a benzene-water solvent
system, and found that it will predominately produce the saturated aldehyde.
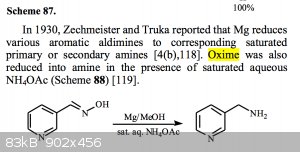
Also, there is the possibility of magnesium in methanol. I have a paper that can be found here on SM, but I have attached for your convenience, that
states oximes can be reduced using magnesium-methanol with saturated aqueous NH4OAc, so what if I exclude the NH4OAc and run the reduction using the
conditions that the paper described to reduce conjugated double bonds? My thinking is that I would form the oxime, reduce, and hopefully the oxime
would be intact, and from there the oxime could be cleaved back to the aldehyde.
You guys are definitely more knowledgeable than I am, and can probably suggest a better solution. So also, what are some suggestions? The only
path I really would like to stay away from is direct hydrogenation using hydrogen gas and expensive catalyst.
Attachment: 14259790.pdf (208kB)
This file has been downloaded 2219 times
Attachment: camps1986.pdf (449kB)
This file has been downloaded 578 times
Attachment: louisandre1985.pdf (125kB)
This file has been downloaded 546 times
[Edited on 30-11-2017 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Couldn't you protect the carbonyl by forming an acetal and then hydrolyze it after the reduction?
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Does an acetal still have the same character as a carbonyl? Would that double bond not then be considered an isolated double bond that is not in
conjugation? Or is the carbonyl simply masked while retaining its character?
I had this thought a while back when I first thought of attempting this reaction, but thought NaBH4 wouldn’t be able to then reduce that double
bond.
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I don't think NaBH4 can reduce that double bond by itself with the acetal in place. I'm actually not 100% sure what could be used to reduce that
double bond other than hydrogenation....
Just guesses:
Sodium might work.
Borohydride with a palladium catalyst might work.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I know that sodium in alcohol can reduce that double bond when it’s conjugated, not sure about when conjugation is lost.
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Nicodem
|
Thread Moved
30-11-2017 at 07:47 |
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
http://orgsyn.org/demo.aspx?prep=cv7p0451

The discussion topic seems to imply that a sodium amalgam reduction of cinnamaldehyde dimethyl acetal will produce hydrocinnamaldehyde dimethyl
acetal. I haven't grabbed the reference yet, but this is a start. I have both sodium and mercury metal, but don't like the idea of using it. I am
willing to consider it. The formation of the amalgam is exothermic, and likely would be better accomplished under an inert atmosphere, but as long as
I go slow it should be fine.
The mentioned reference is as follows.
Dollfus, W. Ber. 1893, 26, 1971.
(probably in German)
[Edited on 30-11-2017 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
laserlisa
Hazard to Self
 
Posts: 52
Registered: 5-2-2016
Member Is Offline
Mood: No Mood
|
|
To avoid messing around too much with protection groups etc, Id reduce it all the way to the alcohol and then oxidize the alcohol back to the aldehyde
in the 2nd step. Maybe with dess martin, swern or tempo oxidation.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I don't really suggest trying this, but you can make sodium amalgam by electrolysis of sodium chloride with a mercury cathode: https://www.youtube.com/watch?v=S4VEZk9ccWg
I'd try to avoid using massive quantities of mercury if possible.
Pretty much any chromium-based oxidizer would work for oxidizing the alcohol to the aldehyde as long as you don't let things get too warm (in which
case the benzylic carbon will oxidize) or too wet (in which case the aldehyde will oxidize to the carboxylic acid). Bipyridyl chromium (VI) oxide
peroxide or PCC would do the trick I think.
[Edited on 30-11-2017 by JJay]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
IIRC the Mg/MeOH reduction of oximes was tried several times and it doesn't work anyway. However I have more confidence in the dithionite/PTC method
for this rxn.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2733
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
A simple hydrogenation is the best way to reduce a double bond in the presence of a carbonyl. Few other methods work as well. Even a simple
transfer hydrogenation should work, a tiny amount of Pd on Carbon would do, then just some sodium formate or one of several others would do. Even
using Raney Nickel and hydrazine might generate the hydrogen you need, only 1 atm is needed for most double bonds.
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
This "review" about Mg/MeOH, from COCh, again returns like shitty boomerang.
And again returns reference [119] about reduction of oximes.
This reference (by Sugden) is posted on the board, as far as remember by Mush, you can search it for yourself.
However, the last sentence from the reference is:
"Cinnamaldoxime and 4-nitrobenzaldoxime
decomposed to intractable tar under the reaction conditions employed."
Back to the matter, what I would do ?
I would try Mg/MeOH reduction, just to see what happens.
Then Na2S2O4, but recently I have read that cinnamic acid (or rather its sodium salt) is inert to dithionite. Some article from Tet.Lett. gives ~100 %
reduction of cinnamate esters for Mg/MeOH system....
But what would be the best ?
As it was mentioned: hydrogen at 1 atm, Pd/alumina/carbon, aldehyde in metanol or other ethanol. Should work perfectly, with yield close to 100 %.
BTW.
I recommend Hudlicky's book about reduction and this: J. Org. Chem., Vol. 43, No. 20,1978 (Palladium-Catalyzed Reductions of a/b-Unsaturated Carbonyl
Compounds, Conjugated Dienes, and
Acetylenes with Trialkylammonium Formates) - no H2 is needed.
[Edited on 30-11-2017 by kmno4]
Слава Україні !
Героям слава !
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Loptr  | | I know that NaBH4 will prefer reduction of the a,b-unsaturated conjugated double bond since it is a softer nucleophile |
NaBH4 will actually reduce the aldehyde rather than the double bond ( cinnamaldehyde to cinnamyl alcohol ) ,instead of the other way round
but LiAlH4 will reduce both the double bond and the aldehyde.
I think the best thing to do would be to reduce it completely to hydrocinnamyl alcohol and then bleach it back to aldehdye,like laserlisa suggested
literally every selective method uses some or the other crazy catalyst or H2 gas-
http://pubs.rsc.org/en/content/articlelanding/1984/c3/c39840...
http://www.sciencedirect.com/science/article/pii/S0022328X09...
https://www.thieme-connect.com/products/ejournals/abstract/1...
http://pubs.rsc.org/en/content/articlelanding/2009/gc/b81556...
[Edited on 1-12-2017 by CuReUS]
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I have read that you will actually end up with a mixture of the two... it just depends on which is attacked first, since both are likely. I have read
conflicting information in chemistry books, where one states it would tend towards one being reduced before the other, and vice-versa. At this point,
I think it's left to experimentation to determine. If I have some time, I will try and find what I am talking about to back up my statements.
There is also the potential of altering the NaBH4 to cause it to attack the carbonyl more slowly, such as using acetic acid, but I am not sure what
effect that has on its ability to reduce that double bond. I have a paper that states it took 40 hours for NaBH(OAc)3 to reduce an aldehyde in 60%
yield. However, I think it's too mild of a reducing agent for this purpose.
I haven't had a chance to read through your links yet.
I was also thinking it might end up being a couple steps to the end product, but wanted to see if there were a more selective reduction method. I am
seriously considering the catalytic hydrogenation route, and seeing if a less expensive catalyst could work. I don't have a lot of time to spend
experimenting, so I like to make heads or tails of what would most likely work before trying anything... a lot of naval gazing, and then
experimentation. I know that I can get to hydrocinnamyl alcohol, and then oxidize it back to the aldehyde, but a single reduction is what I am looking
for.
[Edited on 1-12-2017 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Well, this is certainly interesting. Hydroxylamine hydrochloride and ethyl acetate used for the preparation of diimide. I have read about diimide
reductions before, but thought they required harder to obtain reagents, such as potassium azodicarboxylate.
This might be a viable option. Trying to find an overview of the mechanism now to see if it would have any affect on that aldehyde.
Attachment: gangadhar1984.pdf (343kB)
This file has been downloaded 527 times
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
According to this, there might be some issues with reducing double bonds in a,b-unsaturated carbonyl compounds.

Attachment: miller1965.pdf (4.5MB)
This file has been downloaded 880 times
The easiest method really does seem to be Pd/C and formate. I might be purchasing some in the future. What is the cheapest source?
[Edited on 1-12-2017 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I continue to recommend dithionite/PTC. The objection of kmno4 is not relevant and the literature attached explains why.
| Quote: | | Then Na2S2O4, but recently I have read that cinnamic acid (or rather its sodium salt) is inert to dithionite. Some article from Tet.Lett. gives ~100 %
reduction of cinnamate esters for Mg/MeOH system.... |
This is not too surprising, because dithionite is only reported effective for ketones and aldehydes, not acids. However, it will perform a
regioselective reduction of dienoic acids to allylic acids, that is, it reduces alpha,beta,gamma,delta-unsaturated acids to
beta,gamma-unsaturated acids. See Camps et al 1986 and Camps et al 1982.
Attachment: camps1986.pdf (449kB)
This file has been downloaded 460 times
Attachment: camps1982.pdf (5.3MB)
This file has been downloaded 605 times
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by clearly_not_atara  | I continue to recommend dithionite/PTC. The objection of kmno4 is not relevant and the literature attached explains why.
| Quote: | | Then Na2S2O4, but recently I have read that cinnamic acid (or rather its sodium salt) is inert to dithionite. Some article from Tet.Lett. gives ~100 %
reduction of cinnamate esters for Mg/MeOH system.... |
This is not too surprising, because dithionite is only reported effective for ketones and aldehydes, not acids. However, it will perform a
regioselective reduction of dienoic acids to allylic acids, that is, it reduces alpha,beta,gamma,delta-unsaturated acids to
beta,gamma-unsaturated acids. See Camps et al 1986 and Camps et al 1982. |
Thanks for the info. I will prioritize the dithionite/PTC reduction.
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
There is also this
Reduction to the saturated aldehyde by passing over alumina........ Attached
/CJ
Attachment: Reductions with Alcohols of Cinnamaldehyde to Beta-Phenylpropionaldehyde (1929).pdf (72kB)
This file has been downloaded 498 times
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Could sodium metabisulfite be use to protect the aldehyde?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
According to Hilgetag and Martini's Preparative Organic Chemistry page 6 (in the SM library), sodium and alcohol can reduce the double bond of
cinnamic acid. In the case of cinnamaldehyde, I think the bisulfite adduct would be decomposed by the strongly basic conditions, but that might work
with a different reducing agent.
Interestingly, they cite this paper on poison ivy to back up their claim: http://pubs.acs.org/doi/abs/10.1021/ja01587a025
|
|
|
wxyz
Harmless

Posts: 20
Registered: 13-4-2010
Member Is Offline
Mood: No Mood
|
|
Catalytic hydrogenation with Pd/C will reduce double bonds, usually without affecting aldehydes. One exception is that aldehydes alpha to an aromatic
ring can be reduced by catalytic hydrogenation ... which isn't the case with cinnamaldehyde. And even in that case the double bonds would be reduced
first. But, note that the double bond in this case puts the aldehyde carbon in conjugation with the benzene ring so it may be reducible with catalytic
hydrogenation. So it would be wise to not overdo it. Still, all signs point to the double bond being reduced much more easily by catalytic
hydrogenation.
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Hy @Loptr.
Isn't easier oxidate phenyl propyl alcohol to hidrocinnamaldehyde?
phenyl propyl alcohol is easily find at perfumary stores as an essence and it's very cheap.
You can oxidize it by swern oxidation, PCC or with nickel peroxide in organic media, withouth break the aliphatic chain, to hidrocinnamaldehyde.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
That's a good idea, but my interest wasn't in obtaining hydrocinnamaldehyde. It was more about the regiospecific reduction.
Thanks for the info, though.
I hopefully will have some time to experiment coming up. 
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
According what I have studied, It's not possible to reduce the double bond of cinnamaldehyde without reduce the carbonyl group either.
The opposite is possible, just to reduce the carbonyl group and not the double bond, but to reduce the carbon double bond you necessarily have to
reduce the carbonyl group first.
Then, you can reduce cinnamaldehyde to cinamyl alcohol or go further and reduce it to hidrocinamyl alcool, but it's not possible to reduce only the
double bond to obtain hydrocinnamaldehyde from cinnamaldehyde.
You can do that with borohydride complexes or with LiAlH4. The last has an interesting action upon cinnamaldehyde. "add LAH to cinnamaldehyde and you
get just reduction of the carbonyl group; invert the order of addition and you additionally get reduction of the double bond", the researcher claims:
http://www.ch.imperial.ac.uk/rzepa/blog/?p=13688
(extracted from my post in: http://www.sciencemadness.org/talk/viewthread.php?tid=6380&a...
See also:
F.A. Hochstein, and W.G. Brown, "Addition of Lithium Aluminum Hydride to Double Bonds", J. Am. Chem. Soc., vol. 70, pp. 3484-3486, 1948. http://dx.doi.org/10.1021/ja01190a082
Unfortunately It's impossible for me to give you the answer you're expecting, cause apparently the regioselectivity favours the reduction of the
carbonyl group first, before the double bond reduction, with all modern reagents I researched.
May be exists a way to do what you are asking for, but I humbly admit I don't know how.
Reconsider the phenylpropyl alcohol mild oxidation like I said before if you really want to get hydrocinammaldehyde. If you are only interested in the
regioselectivity, I apologize, but unfortunately I can't help you with this, despite my efforts.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by Chemi Pharma  | According what I have studied, It's not possible to reduce the double bond of cinnamaldehyde without reduce the carbonyl group either.
The opposite is possible, just to reduce the carbonyl group and not the double bond, but to reduce the carbon double bond you necessarily have to
reduce the carbonyl group first.
Then, you can reduce cinnamaldehyde to cinamyl alcohol or go further and reduce it to hidrocinamyl alcool, but it's not possible to reduce only the
double bond to obtain hydrocinnamaldehyde from cinnamaldehyde.
You can do that with borohydride complexes or with LiAlH4. The last has an interesting action upon cinnamaldehyde. "add LAH to cinnamaldehyde and you
get just reduction of the carbonyl group; invert the order of addition and you additionally get reduction of the double bond", the researcher claims:
http://www.ch.imperial.ac.uk/rzepa/blog/?p=13688
(extracted from my post in: http://www.sciencemadness.org/talk/viewthread.php?tid=6380&a...
See also:
F.A. Hochstein, and W.G. Brown, "Addition of Lithium Aluminum Hydride to Double Bonds", J. Am. Chem. Soc., vol. 70, pp. 3484-3486, 1948. http://dx.doi.org/10.1021/ja01190a082
Unfortunately It's impossible for me to give you the answer you're expecting, cause apparently the regioselectivity favours the reduction of the
carbonyl group first, before the double bond reduction, with all modern reagents I researched.
May be exists a way to do what you are asking for, but I humbly admit I don't know how.
Reconsider the phenylpropyl alcohol mild oxidation like I said before if you really want to get hydrocinammaldehyde. If you are only interested in the
regioselectivity, I apologize, but unfortunately I can't help you with this, despite my efforts. |
Thank you for the feedback, Chemi Pharma. I appreciate your efforts.
I have made a bit of headway in my research since this thread. I think I have some references that refer to solvent effects on the reduction of
cinnamaldehyde with NaBH4 to favor 1,4-reduction instead of 1,2-reduction. There is also the possibility of altering the activity of NaBH4 so that it
attacks the carbonyl group more slowly, such as is the case with Na(CH3COO)3BH, where it can take days for completion.
I also have a parallel effort where I am researching a catalytic transfer hydrogenation with a nickel metal catalyst supported on activated charcoal.
I posted pictures in the hydrogenation thread currently in Today's Posts. I am not interested in a full-on hydrogenation, but am rather interested in
an activated nickel catalyst with another hydride donor, such as ammonium formate, formic acid, etc. I am aiming for atmospheric pressure reaction
conditions. Pyridine can be added to a nickel catalyst to keep it from reducing the aldehyde.
[Edited on 1-8-2018 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
| Pages:
1
2 |