VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
Protein Assay of Glycine Max Grown in Martian Soil Analog and Earth Control
This is some data from my science fair project this year: Nutrition and Cobalt Content of Glycine Max (Soybeans) Cultivated in Martian Soil Analog- A
Three Year Study. This is a continuing three year research project of mine I started when I was a freshman in High School and is intended to evaluate
the possibility of using Martian regolith as a growing medium for a colonization effort's agriculture.
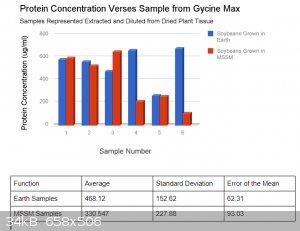
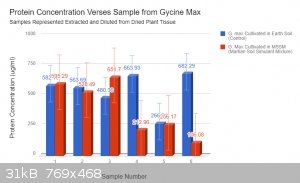
The data bellow this is the protein concentration of an extract prepared from both G. max grown in a Martian Soil Stimulant Mixture and Earth control
(potting soil, no additives). The samples were prepared by centrifuging .1g of dried leaf with 2ml of dH2O. The absorbance was read using a
spectrometer and protein indicator. This absorbance was used to arrive at a protein concentration (ug/mL) using a standard curve and linear
regression. What do you guys think? I am collecting more data tomorrow from more samples. Blue represents earth samples and red martian.
Last year I received 1st place at districts and 4th place at states. This year I intend to win 1st and proceed to nationals. This data is the rough
draft of the actual graph and chart. I was hoping to write a paper on this and publish it on this site once I finish the project.
[Edited on 16-11-2017 by VSEPR_VOID]
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Potting soil no additives could be anything, shouldnt you name the brand and source? Also details of martian mix? Where did you get the data from, for
the martian mix?
Why the big difference in samples? You didnt explain enough to really understand what the data is your showing. Apart from that....
Good luck with it, let us know how it goes.
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
As far as statistical analysis and all the other details this is just a bit of data I wanted to share and not the full project. I am in the process of
writing up the paper describing my procedure, materials, data. The Martian soil actually came from Orbital Technologies Corporation and is referred to
as JSC-1A. I use my own acronym because we have to present to many judges and it helps to make things easy to digest. Its far simpler to tell a judge
that its a martian soil stimulant than to go through all the specs and product details.
JSC-1A was originally designed to be share physical and chemical characteristics with Martian regolith for use in everything from testing rovers to
creating Mars like environments. Its composed mainly of metal and semi metal oxides. In particular, silicon dioxide, titanium dioxide, and iron
oxides.
The data is my own. After cultivating the soybeans I extracted the soluble proteins and used a indicator to allow for VIS. Using the absorption and a
standard curve for I was able to find the concentration of soluble proteins. Well there is a procedure for extracting and marking insoluble proteins I
don't have the amount of time in the lab to do so 
https://en.wikipedia.org/wiki/Martian_regolith_simulant
[Edited on 16-11-2017 by VSEPR_VOID]
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by VSEPR_VOID  | As far as statistical analysis and all the other details this is just a bit of data I wanted to share and not the full project. I am in the process of
writing up the paper describing my procedure, materials, data. The Martian soil actually came from Orbital Technologies Corporation and is referred to
as JSC-1A. I use my own acronym because we have to present to many judges and it helps to make things easy to digest. Its far simpler to tell a judge
that its a martian soil stimulant than to go through all the specs and product details.
JSC-1A was originally designed to be share physical and chemical characteristics with Martian regolith for use in everything from testing rovers to
creating Mars like environments. Its composed mainly of metal and semi metal oxides. In particular, silicon dioxide, titanium dioxide, and iron
oxides.
The data is my own. After cultivating the soybeans I extracted the soluble proteins and used a indicator to allow for VIS. Using the absorption and a
standard curve for I was able to find the concentration of soluble proteins. Well there is a procedure for extracting and marking insoluble proteins I
don't have the amount of time in the lab to do so 
https://en.wikipedia.org/wiki/Martian_regolith_simulant
[Edited on 16-11-2017 by VSEPR_VOID] |
I Hope you didnt take what i said the wrong way, dont forget we only know what you show. Interesting about the martian soil though, a bit presumptuous
of them though. A bit like landing in London and assuming the entire UK is concrete lol.
Look forward to reading it, just make sure you are super anal and cross your I's etc etc. Can you get after school access to the lab for a one off to
grab the protein data? Seems a bit unfair to do all that work over an extended period, and for the sake of a few hours, not let you grab all the data
you can.
Interesting project though.
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
What sort of protein assay are you using? They are often highly sensitive to interference or give wildly different results based on the particular
protein being observed.
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
Quote: Originally posted by UC235  | | What sort of protein assay are you using? They are often highly sensitive to interference or give wildly different results based on the particular
protein being observed. |
Your right, many different assays test for different proteins.
http://www.sigmaaldrich.com/catalog/product/sigma/tp0200?lan...
This is the particular kit I have violable. I have room for possible error. The protein standard I used to make my standard curve will not perfectly
align with the proteins naturally found in soybeans.
As for the time crunch my project is due at December and I work on this between classes and at lunch. It takes a large amount of time to prepare
samples, run the spec, clean glassware ect... I do many chores around my schools lab in return for all the work my teacher allows me to do.
In addition, last year some of my plants cultivated in MSSM (Rye Grass) tested positive for cobalt in an unknown concentration. I did a similar test
this year with the soybeans and found the cobalt concentrations to be nominal. This means either one of the test's results were wrong or the soybeans
and rye absorb cobalt at different rates. Does anyone know why this may be?
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Colbalt uptake decreases as PH rises, but not sure on absorption rates between grasses and Soybeans.
|
|
|
Metacelsus
International Hazard
    
Posts: 2554
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
It might also be good to investigate the perchlorate levels in the soybeans (assuming your Martian soil simulant has perchlorate, which it should have
if it is accurate).
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
Quote: Originally posted by Metacelsus  | | It might also be good to investigate the perchlorate levels in the soybeans (assuming your Martian soil simulant has perchlorate, which it should have
if it is accurate). |
This particular analog lacks perchorate salts. I am considering for next year adding an appropriate amount of potassium perchlorate and finding an
efficient and cost effective way to remove it. Perchorates can chemicals burn the roots most plants and pose a serious health risk. There is some
evidence that the regolith on Mars also contains chromates. My high school's lab lacks the safety equipment to allow me to feel comfortable working
with large amounts of an chromate salts.
The data from which the Martian soil analog was developed comes from three separate rovers all in different locations. Orbiting satellites also have
shown that about of fifth of the permafrost by mass should be water ice along with some carbon dioxide ice. The data from Curiosity supports this.
As a side note it would be interesting if someone started doing speculative more biochemical work for environments like Titan and Europa. In a book I
am currently reading, "All These Worlds Are Yours" the author focuses more on Titan as a possible harbor for life than Mars or Europa.
An organism's biochemistry on Titan would wildly differ from that of Earth, using liquid ammonia and methane as a solvent rather than water. Chemical
reactions would take much longer as well. Research indicates that it is very possible to enzymes to function even at -100C if they are in an aqueous
environment and have abscess to the necessary co enzymes, co factors, and energy carrying molecule.
Perhaps life on Titan would using hydrogen bonding and dispersion forces much more than Terran life. At lower temperatures such bonds would be more
stable.
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Why stop there, there is a number of planets said to be in the goldilocks zone.
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
More data: It was all collected using the same manor and procedure.
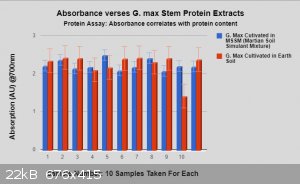 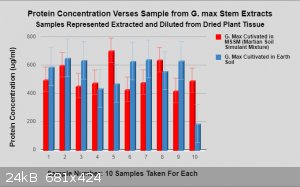
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|