HenningBasse
Harmless

Posts: 12
Registered: 22-1-2007
Location: Earth\'s Bottom
Member Is Offline
Mood: XTC
|
|
can this work??[ammonia related]
so , i've setup a little lab with few glassware and reagents , and i wanna make ammonia solution (like NH4Cl or NH4OH) to make some qualitative
analysis
so , here are my thoughts about my recent experiment
Urea + NaOH (6 molar or more) +H2O (potable water)--heat-on-baker-->NH3(g) + NH4+(l) + NH2COO- + H2O
strong ammonia odor was released
then i added some 6 M HCl , got some redish brown precipitate (i tought it was some like NH2COOH) , and a strong ammonia smell decantate...so i
filtered it and got this "ammonia liquid" .....but maybe its mixed with some urea that wasn't reacted with OH....
can u help me about key reaction CO(NH2)2 + OH --->NH3 + NH2COOH?? it's the right and unique way??? please some analytical and inorganic chemists
help me out!! thx anyway
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Welcome to Science Madness.
Please check the Search feature before posting; this can get you to what you want much faster. Since direct NH3 from urea and the reactions involved
have not to my knowledge been addressed, I'll let you off the hook . .
Another thread dealing with ammonia generation/concentration is here:
http://www.sciencemadness.org/talk/viewthread.php?tid=7464#p...
I'll also add that NaOH + amino acid + heat (glutamine, for example) will also yield NH3 (see deamidation). A paper describing the original urea assay
is attached.
Cheers,
O3
Attachment: Urea Assay_01.pdf (599kB)
This file has been downloaded 870 times
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
HenningBasse
Harmless

Posts: 12
Registered: 22-1-2007
Location: Earth\'s Bottom
Member Is Offline
Mood: XTC
|
|
i realized that there's no NH2COOH precipitate since at low pH , the amine is already protonated NH3+COOH , so its quite soluble in acidic solution
(damn , feel bad for pass out that fault)
|
|
|
HenningBasse
Harmless

Posts: 12
Registered: 22-1-2007
Location: Earth\'s Bottom
Member Is Offline
Mood: XTC
|
|
ehh , yeah , sorry about that Ozone , but really im not a new user here , this is just a new account...but anyway i must entered to some "welcome"
forum....
|
|
|
HenningBasse
Harmless

Posts: 12
Registered: 22-1-2007
Location: Earth\'s Bottom
Member Is Offline
Mood: XTC
|
|
a more theoretical approach
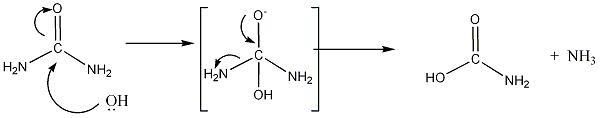
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
| Quote: | Originally posted by HenningBasse
a more theoretical approach
 |
It should be NH2COO- + NH3
[Edited on 2/6/2007 by guy]
|
|
|
HenningBasse
Harmless

Posts: 12
Registered: 22-1-2007
Location: Earth\'s Bottom
Member Is Offline
Mood: XTC
|
|
| Quote: | Originally posted by guy
| Quote: | Originally posted by HenningBasse
a more theoretical approach
 |
It should be NH2COO- + NH3
[Edited on 2/6/2007 by guy] |
yeah , that's right , just i did it quickly on chemdraw 
|
|
|